Between 1961 and the late twentieth century, major drug safety failures resulted in thousands of preventable adverse outcomes worldwide, fundamentally changing how medicines are regulated after approval. These events demonstrated that pre-marketing testing alone could not protect patients once products entered widespread clinical use. They forced regulators to introduce continuous safety monitoring and reshaped how inspections evaluate post-marketing controls. This shift laid the foundation for modern Pharmacovigilance and explains why safety oversight is now examined with the same rigor as manufacturing quality.
The history of pharmacovigilance did not develop as an academic exercise, but as a regulatory response to real patient harm. For pharmacovigilance professionals, regulatory affairs teams, QA staff, and life-science students, this background is not optional context. It explains why inspections today focus so heavily on signal management, documentation discipline, and governance maturity.
Table of Contents
What Is Pharmacovigilance in a Regulatory Context
Pharmacovigilance is the regulated system that ensures a medicine’s benefits continue to outweigh its risks throughout real-world use, not just at the time of approval.
For regulators and inspectors, pharmacovigilance is not a reporting activity but a quality-critical system. During inspections, authorities examine how safety information is captured, medically assessed, escalated, and converted into documented decisions. This is why pharmacovigilance is reviewed alongside GMP systems as a core indicator of whether a company can maintain regulatory control after a product reaches patients.
Why the Pharmacovigilance History Matters for GMP Compliance
Modern GMP expectations are a direct consequence of past drug safety failures. The history of pharmacovigilance explains why regulators no longer tolerate informal safety oversight or undocumented medical judgment. Repeated crises showed that signals often existed but were missed due to weak systems, unclear accountability, or delayed decision-making.
As a result, pharmacovigilance requirements evolved in parallel with GMP principles. Today, inspectors expect safety monitoring to function as a controlled system with defined roles, training, documentation, and management oversight. Many critical inspection findings arise not from scientific disagreement, but from quality system weaknesses within pharmacovigilance operations.
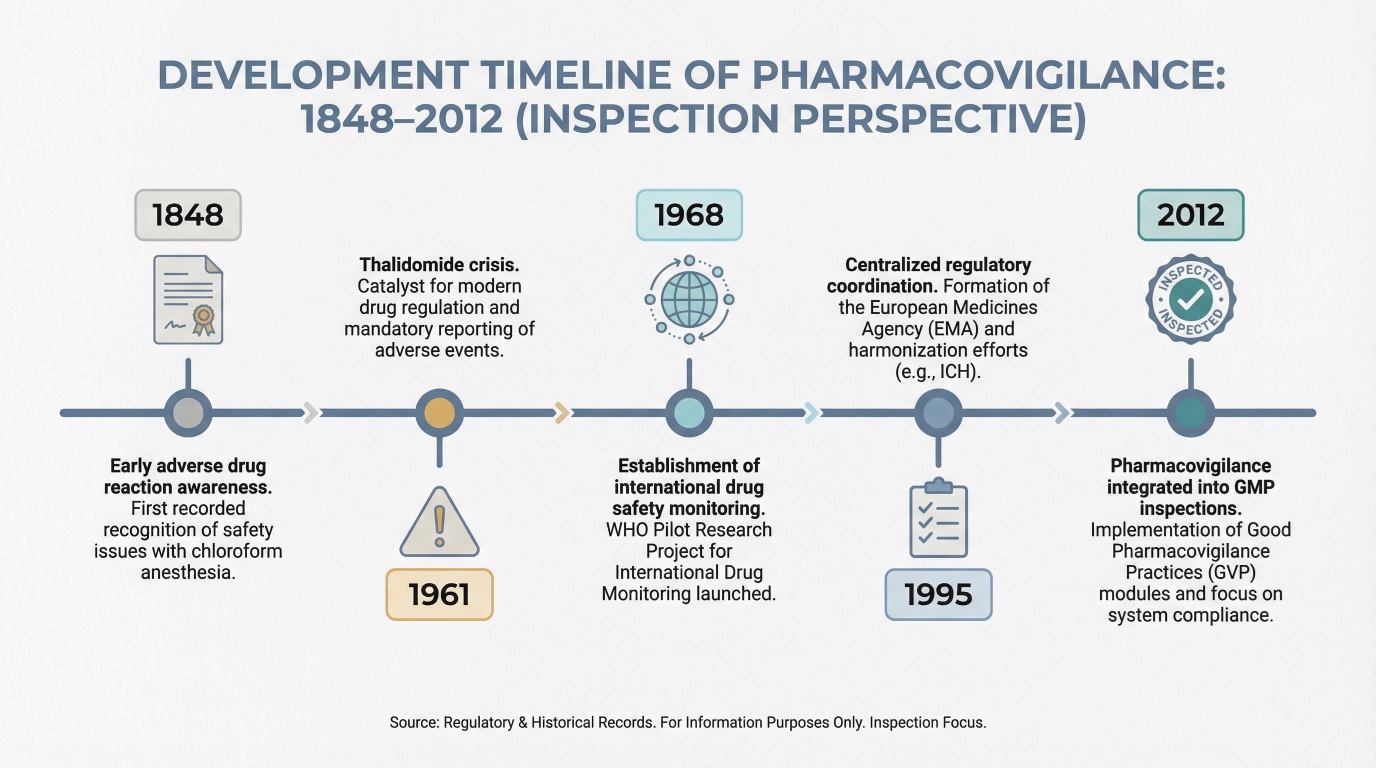
Key Milestones in the Evolution of Pharmacovigilance
Today’s inspection focus reflects lessons learned from historical system failures.
From a regulatory and GMP inspection perspective, pharmacovigilance did not evolve linearly or proactively. It developed through a series of critical failures that exposed structural weaknesses in drug safety oversight. Each major milestone represents a regulatory response to a specific breakdown missed signals, delayed action, lack of accountability, or inadequate post-marketing controls.
For inspectors today, these milestones are not historical footnotes. They define why pharmacovigilance systems are evaluated as quality-critical, why governance and documentation are scrutinized, and why companies are expected to demonstrate control, traceability, and decision-making maturity across the entire safety lifecycle.
We will discuss:
- Early Drug Safety Incidents and Regulatory Awareness (1848–1960)
- The Thalidomide Crisis and Global Regulatory Reform (1961–1968)
- Development of Formal Pharmacovigilance Systems (1968–1995)
- Integration of Pharmacovigilance into GMP and Inspections (1995–2012)
Early Drug Safety Incidents and Regulatory Awareness (1848–1960)
Before formal pharmacovigilance existed, drug safety oversight relied largely on professional ethics and fragmented national responses. Early adverse drug reactions revealed that medicines could cause serious harm long after approval, yet there were no standardized mechanisms to capture or analyze these events.
This period gradually increased regulatory awareness but lacked enforceable post-marketing obligations. For inspectors today, it illustrates why voluntary safety monitoring is insufficient once products are widely used across populations.
The Thalidomide Crisis and Global Regulatory Reform (1961–1968)
The thalidomide crisis, caused by the widespread use of thalidomide as a sedative and anti-nausea drug during pregnancy in the late 1950s and early 1960s, resulted in severe congenital malformations in thousands of newborns worldwide. This event marked a decisive turning point, demonstrating that drug safety could not be assumed after approval and that systematic post-marketing surveillance was essential.
Regulatory reforms during this period strengthened approval standards and formalized adverse reaction reporting. International collaboration on drug safety emerged, laying the groundwork for pharmacovigilance as a regulatory discipline rather than an informal professional practice.
Development of Formal Pharmacovigilance Systems (1968–1995)
From the late 1960s through the mid-1990s, pharmacovigilance matured into a structured global activity. National centers were established, reporting standards evolved, and early signal detection methodologies were introduced.
During this era, safety data shifted from isolated case reports to analyzable datasets supporting regulatory action. This transition also introduced inspection expectations that companies must demonstrate how safety data informed regulatory and medical decisions.
Integration of Pharmacovigilance into GMP and Inspections (1995–2012)
As regulatory frameworks expanded, pharmacovigilance became embedded within quality and inspection models. Safety monitoring was no longer separate from compliance; it became a core element of regulatory oversight.
Inspectors increasingly evaluated system design, training effectiveness, vendor oversight, and documentation control. In real inspections, companies are often required to reconstruct safety decisions end-to-end, demonstrating how signals were identified, assessed, escalated, and resolved within controlled timelines.
How Historical Lessons Shape Modern Pharmacovigilance Inspections
Inspectors apply historical lessons as a risk filter when evaluating current systems.
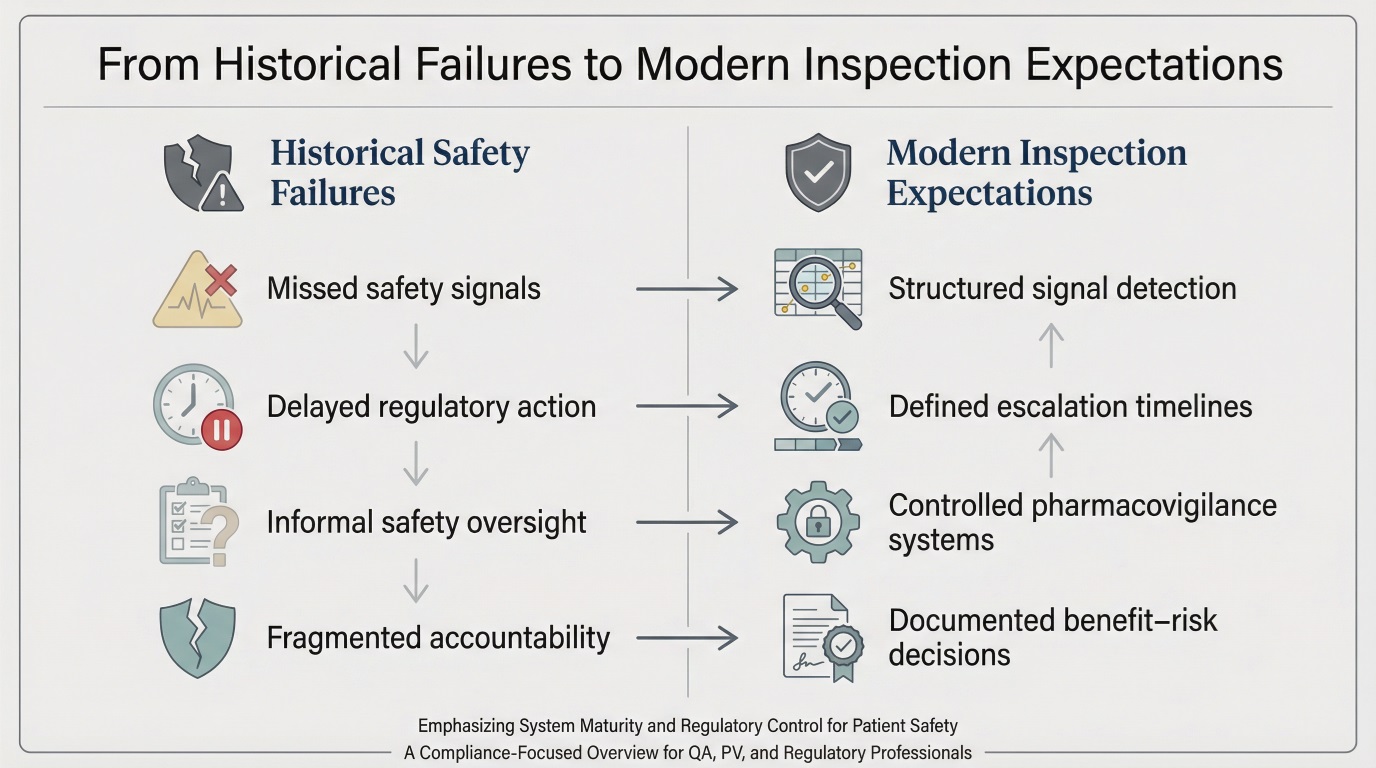
The pharmacovigilance history of past failures explains why inspections emphasize system robustness rather than individual case outcomes. Regulators assess whether known failure modes missed signals, unclear governance, weak oversight of partners have been structurally addressed.
Inspection focus typically includes:
- End-to-end traceability of safety signals
- Clear governance and escalation pathways
- Controlled documentation and data integrity
- Effective oversight of outsourced activities
- Documented benefit–risk decision-making
These expectations are rooted in decades of regulatory learning rather than theoretical best practice.
Final Word
Today, over 160 national authorities participate in global drug safety monitoring programs, showing how central post-marketing oversight has become in pharmaceutical regulation. As a result, regulatory inspections increasingly treat pharmacovigilance systems as quality-critical, applying scrutiny comparable to core GMP operations.
The pharmacovigilance history of past regulatory failures demonstrates a clear pattern: when safety systems break down, inspection intensity rises and regulatory expectations tighten. For pharmaceutical organizations, understanding this trajectory is essential to controlling inspection risk, maintaining compliance credibility, and protecting patients in real-world use.
FAQs
Because historical drug safety failures showed that weak pharmacovigilance systems allow serious patient harm to persist after approval, making PV a core compliance risk during GMP inspections in the pharmaceutical industry.
They forced regulators to require structured post-marketing surveillance, documented benefit–risk decisions, and inspection-ready pharmacovigilance systems as mandatory obligations for pharmaceutical manufacturers.
It defines inspection priorities: regulators assess whether pharma companies have system-level controls that would have prevented past safety failures, not just whether individual cases were processed correctly.

Mahtab Shardi
Mahtab is a pharmaceutical professional with a Master’s degree in Physical Chemistry and over five years of experience in laboratory and QC roles. Mahtab contributes reliable, well-structured pharmaceutical content to Pharmuni, helping turn complex scientific topics into clear, practical insights for industry professionals and students.

Pharmaceutical Warehouse in 2026: GMP Storage Requirements Guide
Effective drug storage compliance depends on validated storage conditions for pharmaceuticals, structured segregation control, and reliable batch traceability in warehouse operations. This guide explains how temperature excursion management and GDP compliance warehouse practices strengthen inspection readiness and regulatory defensibility.
GMP Interview in 2026: Questions and Answers with Audit Insight
More than 60% of regulatory observations in pharmaceutical manufacturing relate to documentation control, investigation quality, and procedural compliance gaps. GMP Interview preparation in regulated environments determines whether a candidate can operate safely under inspection pressure.

Harmonization in pharmacovigilance in 2026
Inspection trends show that inconsistent global safety practices remain a leading source of pharmacovigilance findings. This article explains how regulatory harmonization, aligned safety reporting, and coordinated oversight shape inspection outcomes and support compliant international pharmacovigilance operations.