Career change in pharma has become increasingly common as the pharmaceutical industry expands far beyond traditional laboratory-based roles. Recent workforce data from Europe and North America show that more than 40% of new hires in quality, regulatory, and clinical operations now come from non-laboratory backgrounds. This shift reflects how regulation, systems, and compliance increasingly shape pharmaceutical careers.
Professionals enter pharmaceutical roles from a wide range of university disciplines, including pharmacy, chemistry, biology, biotechnology, biomedical sciences, engineering, information technology, data and statistics, healthcare, and business or management studies. As a result, moving into pharma today feels less like a risky leap and more like a structured, skills-driven transition. Many professionals begin by exploring a defined Pharma Career Path that aligns existing experience with regulated industry expectations.
Table of Contents
Why Professionals Consider a Career Change in Pharma
Professionals from academic backgrounds such as life sciences, engineering, IT, data, healthcare, and business disciplines increasingly consider entering pharma because the industry rewards structured thinking, compliance awareness, and transferable skills alongside scientific knowledge.
Beyond academic background, several structural factors make pharma attractive to career changers. Compared to less regulated industries, pharma offers clarity, predictability, and long-term relevance.
Key motivations include:
- Stable demand driven by global healthcare needs
- Regulation-based structures that define responsibilities clearly
- Long-term career development across multiple functions
- Opportunities to work in global, highly structured organizations
Therefore, pharma attracts professionals who value impact, stability, and professional longevity.
Stability and Long-Term Career Growth
Pharmaceutical companies operate under continuous regulatory oversight. Consequently, organizations invest heavily in quality systems, documentation frameworks, and workforce development. Career paths evolve steadily because regulatory expectations rarely disappear or reset.
Moreover, regulatory complexity increases over time. As a result, professionals who understand regulated environments often enjoy long-term relevance and reduced career volatility compared to less structured sectors.
Transferable Skills That Pharma Values
Pharma does not hire solely based on scientific specialization. Instead, it actively values skills that transfer from other regulated or process-driven industries.
Common skill transfers include:
- Project management → Clinical research coordination and study operations
- IT and data handling → Validation, compliance, and data-integrity roles
- Process improvement → Quality assurance and continuous improvement functions
- Documentation and auditing → Regulatory affairs and quality systems support
- Risk analysis → GMP-driven compliance and inspection-readiness roles
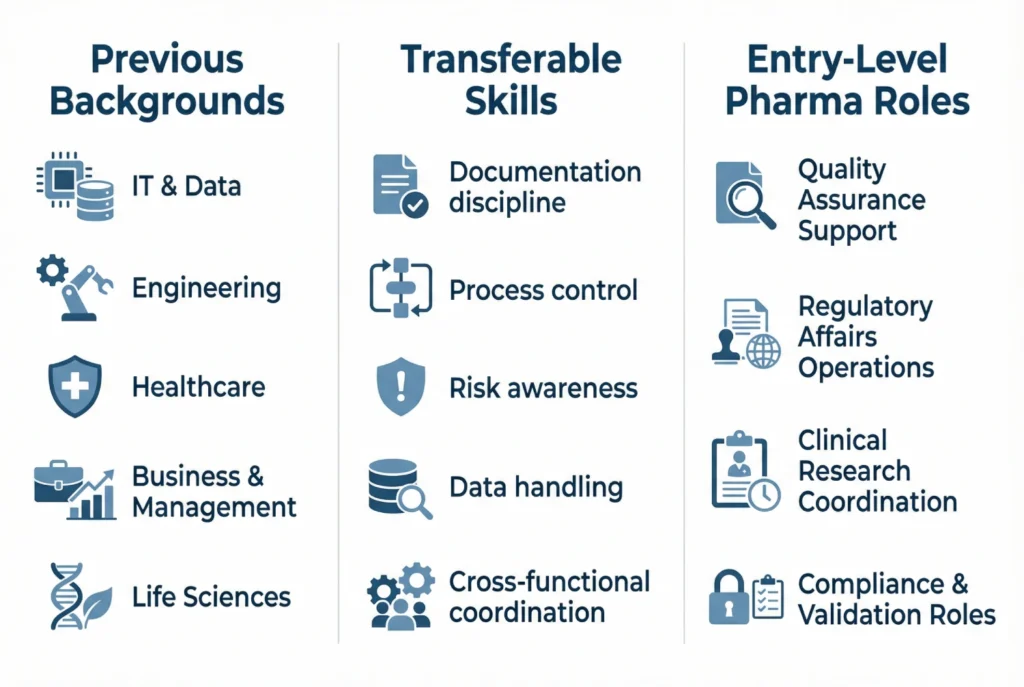
As a result, many professionals realize that their existing experience already matches pharma requirements, even without prior industry exposure.
Common Misconceptions About Entering Pharma
Despite growing demand, misconceptions still discourage professionals from exploring pharma careers. However, these myths often reflect outdated views of the industry.
Common misconceptions include:
- Pharma only hires scientists and lab specialists
- Career changers must start from entry-level positions
- Non-pharma experience holds little value
In reality, pharma relies on diverse non-lab roles that support regulated systems, coordination, and oversight.
Do You Need a Pharma Degree to Switch Careers?
No. While life-sciences degrees remain valuable, pharmaceutical organizations regularly hire professionals from engineering, IT, healthcare, data, and business backgrounds. Experience, mindset, and transferable skills often outweigh formal titles.
Is Lab Experience Mandatory for Pharma Roles?
No. Many pharmaceutical roles exist entirely outside laboratory environments. Quality assurance, regulatory affairs, clinical research coordination, and medical affairs focus on systems, documentation, and compliance rather than bench work.
How Important Is GMP Knowledge for Career Changers?
GMP knowledge significantly improves credibility. It helps professionals understand expectations, terminology, and workflows. However, GMP does not act as a strict barrier to entry. Instead, it accelerates onboarding and integration.
Entry-Level vs Transition Roles: What to Expect
Most career changers do not start from zero. Instead, they enter through transition roles that balance prior experience with pharma-specific learning. Titles may sound junior, but responsibilities often reflect mid-level expectations.
Timeline: How Long Does a Pharma Career Transition Take?
Most professionals complete a pharma transition within 6 to 18 months. The timeline depends on background, role selection, and preparation strategy rather than academic degree alone.
Practical Career Paths for a Career Change in Pharma
Successful transitions often happen when professionals target realistic, system-oriented roles that value transferable skills over laboratory experience.
Below are common transition paths observed across the industry.
Previous Background | Entry Pharma Roles | Key Skills Used |
IT / Data | Validation, compliance roles | Systems, data integrity |
Engineering | Quality assurance, process support | Process control |
Healthcare | Clinical research, medical affairs | Clinical knowledge |
Business / Management | Regulatory coordination | Documentation, communication |
Life sciences | QA, regulatory support | GMP, scientific literacy |
For example, many IT professionals enter pharma through computer system validation or compliance roles, where documentation discipline and risk awareness matter more than laboratory techniques.
How to Prepare for a Career Change in Pharma
Preparation determines success more than background. Therefore, professionals benefit from targeted preparation rather than broad retraining.
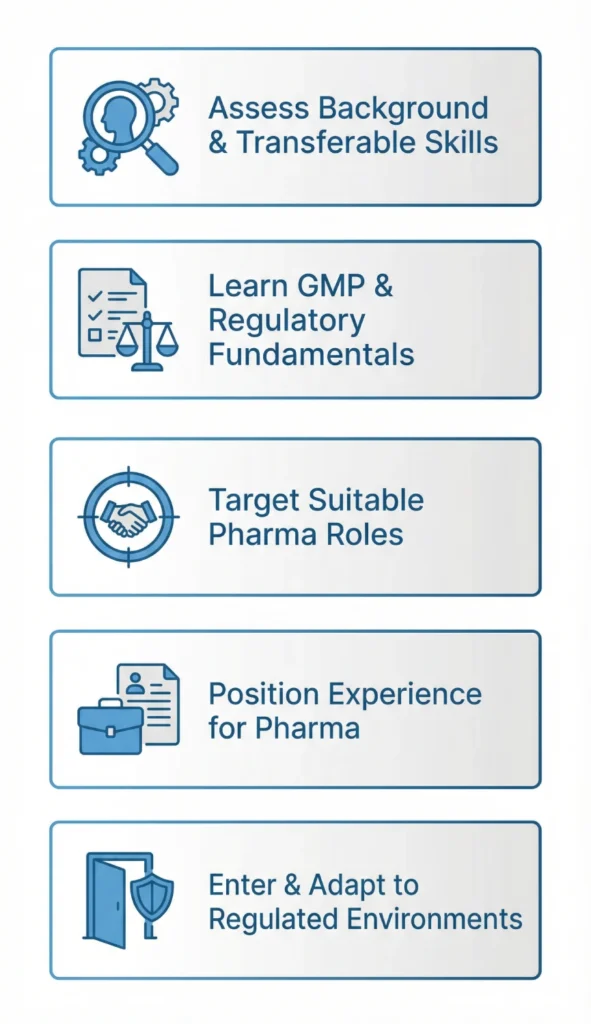
Key preparation steps include:
- Learning GMP fundamentals and regulatory language
- Mapping transferable skills to pharma functions
- Building a pharma-focused CV that highlights compliance exposure
- Developing structured thinking and documentation discipline
- Positioning experience toward regulated environments
As a result, professionals reposition themselves strategically rather than reinventing their careers.
Final words
Career change in pharma is no longer an exception. Industry analyses now show that nearly 50% of new roles in quality, regulatory, and clinical operations are filled by professionals transitioning from other industries.
Because pharma rewards structured thinking, compliance awareness, and transferable skills, professionals can move through clear and realistic pathways instead of uncertain leaps. If you want to reduce career risk and move with confidence, now is the right time to explore pharma career paths.
FAQs:
Yes. Many pharmaceutical roles focus on quality systems, regulatory compliance, clinical coordination, and cross-functional operations rather than laboratory work. As a result, professionals from IT, healthcare, business, and engineering backgrounds succeed in pharma environments where documentation discipline, process control, and regulatory awareness matter most.
Quality assurance support, regulatory affairs operations, clinical research coordination, and compliance-focused roles suit career changers best. These positions emphasize structured workflows, documentation, and coordination skills rather than deep laboratory specialization, which makes them more accessible entry points into the pharmaceutical industry.
GMP experience strengthens professional credibility and reduces onboarding time in pharmaceutical organizations. It helps career changers understand regulatory expectations, documentation standards, and controlled workflows, which allows them to integrate faster into GMP-regulated environments across manufacturing and clinical functions.
Not necessarily. While prior experience in regulated sectors such as healthcare, medical devices, or food can be helpful, many professionals enter pharma without it. What matters is the ability to adapt to GMP-driven processes, documentation requirements, and compliance-focused decision-making common in pharmaceutical organizations.
In most cases, a structured transition into the pharmaceutical industry takes between 6 and 18 months. The timeline depends on background, target role, and preparation strategy, including learning GMP fundamentals and positioning transferable skills for regulated environments.
References
- ISPE – Careers in the Pharmaceutical Industry
https://ispe.org/career-development - FDA – Working in Pharmaceutical Quality
https://www.fda.gov/drugs/pharmaceutical-quality-resources - EMA – Careers and Regulatory Roles in Pharma
https://www.ema.europa.eu/en/about-us/careers

Mahtab Shardi
Mahtab is a pharmaceutical professional with a Master’s degree in Physical Chemistry and over five years of experience in laboratory and QC roles. Mahtab contributes reliable, well-structured pharmaceutical content to Pharmuni, helping turn complex scientific topics into clear, practical insights for industry professionals and students.

Pharmaceutical Warehouse in 2026: GMP Storage Requirements Guide
Effective drug storage compliance depends on validated storage conditions for pharmaceuticals, structured segregation control, and reliable batch traceability in warehouse operations. This guide explains how temperature excursion management and GDP compliance warehouse practices strengthen inspection readiness and regulatory defensibility.
GMP Interview in 2026: Questions and Answers with Audit Insight
More than 60% of regulatory observations in pharmaceutical manufacturing relate to documentation control, investigation quality, and procedural compliance gaps. GMP Interview preparation in regulated environments determines whether a candidate can operate safely under inspection pressure.

Harmonization in pharmacovigilance in 2026
Inspection trends show that inconsistent global safety practices remain a leading source of pharmacovigilance findings. This article explains how regulatory harmonization, aligned safety reporting, and coordinated oversight shape inspection outcomes and support compliant international pharmacovigilance operations.