Recent inspection data highlights a persistent pattern across pharmaceutical manufacturing sites. Between 2020 and 2023, FDA Form 483 summaries indicate that approximately 35–40% of manufacturing-related observations were linked to human error, inadequate procedural execution, or weak deviation handling rather than equipment or facility failures. In many cases, these findings resulted in delayed batch release, repeat observations, or extended regulatory oversight.
As a result, upskilling for pharma manufacturing has emerged as a critical GMP risk-control strategy rather than a discretionary training activity. Regulators increasingly assess whether personnel can apply GMP principles under real operating conditions, not simply whether training records exist. Therefore, structured competency development supported by frameworks such as the Pharma Skill Tree plays a direct role in inspection readiness, patient safety, and sustainable pharmaceutical quality management.
Table of Contents
What does upskilling mean in pharma manufacturing
Upskilling in pharma manufacturing refers to the systematic development of GMP-critical competencies required to perform manufacturing activities correctly, consistently, and compliantly. Unlike generic training, upskilling focuses on demonstrated capability rather than completed learning events.
In regulated environments, upskilling means ensuring that personnel can apply GMP principles under real production conditions, respond appropriately to deviations, and make quality-aligned decisions without supervision. Therefore, effective upskilling is directly linked to manufacturing performance, deviation prevention, and inspection readiness.
Best methods to upskill pharma manufacturing skills
Effective upskilling in regulated manufacturing environments relies on methods that move beyond theoretical GMP training and focus on demonstrated capability. Upskilling for pharma manufacturing succeeds when GMP knowledge is translated into consistent execution under real production conditions. Proven approaches combine structured learning with supervised, real-condition execution to build manufacturing competency pharma and reduce compliance risk.
The most successful programs integrate classroom or digital learning with supervised shop-floor application, allowing personnel to translate GMP principles into controlled actions. This blended approach improves right-first-time manufacturing, strengthens deviation prevention, and supports inspection readiness by providing evidence of sustained performance rather than one-time training completion.
Well-designed upskilling methods also emphasize feedback loops, qualification checkpoints, and performance review under actual production pressures. As a result, organizations create a workforce that not only understands GMP requirements but can consistently apply them during routine operations, deviations, and regulatory inspections.
We will discuss the following approaches:
- Online GMP and pharma manufacturing courses
- On-the-job shop-floor training in pharma manufacturing
- Professional networks and industry communities
- Digital self-learning and regulatory updates
Online GMP and pharma manufacturing courses
Structured online programs provide foundational knowledge in GMP training manufacturing, aseptic processing, contamination control, and deviation handling. When designed correctly, these courses support standardized understanding across teams and sites.
On-the-job shop-floor training in pharma manufacturing
Practical shop-floor training remains the most effective way to build manufacturing competency pharma. Mentored execution, supervised operations, task qualification, and performance observation under real conditions allow organizations to verify that skills translate into compliant behavior.
Professional networks and industry communities
Webinars, expert forums, and peer discussions expose manufacturing teams to real inspection scenarios and evolving regulatory expectations. As a result, professionals gain contextual understanding beyond internal procedures.
Digital self-learning and regulatory updates
Continuous self-learning through inspection reports, regulatory guidance, and industry publications helps personnel remain aligned with current expectations. This approach supports regulatory readiness workforce development and reduces knowledge decay between inspections.
Where pharma manufacturing skills gaps impact GMP compliance most
Skill gaps most frequently affect GMP compliance in high-risk manufacturing areas, including:
- Aseptic processing and contamination control
- Deviation investigation and root cause analysis
- Equipment setup, changeover, and cleaning verification
- Documentation accuracy and real-time data recording
- Batch release decision-making under pressure
In these areas, insufficient competency often leads to repeat deviations, inspection observations, and delayed approvals.
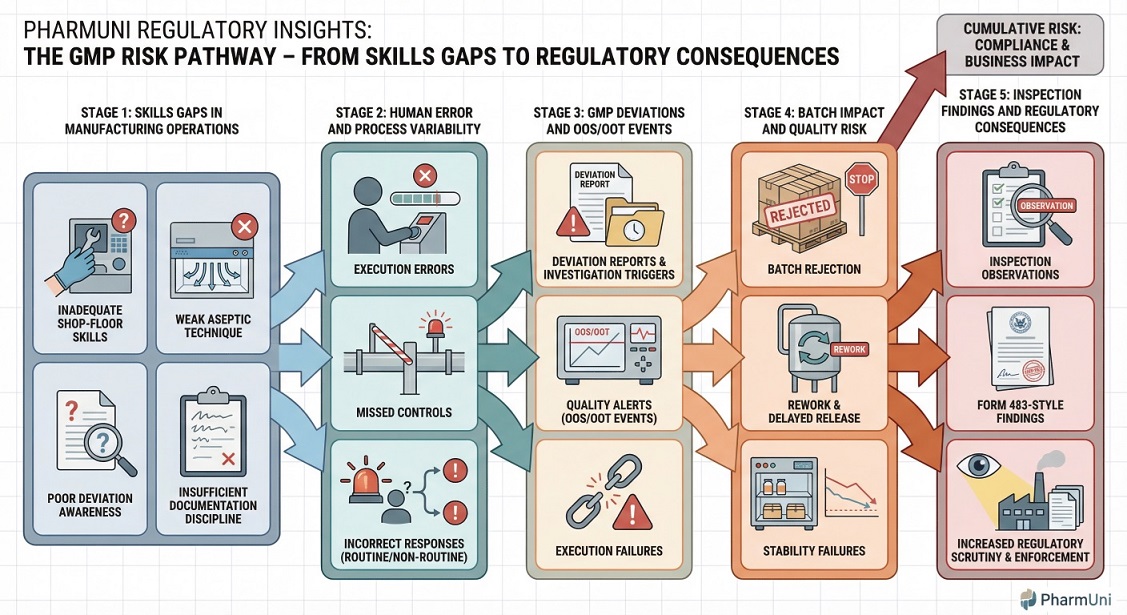
Human error caused by missing shop-floor skills in pharma manufacturing
From an inspection perspective, upskilling for pharma manufacturing directly addresses recurring human errors by strengthening shop-floor decision-making and execution under GMP conditions.
Regulators rarely accept “human error” as a standalone explanation. Instead, inspection findings typically interpret human error as evidence of missing shop-floor skills or weak competency systems. When personnel lack practical understanding of process risks, errors become predictable outcomes rather than isolated events. Consequently, deviation reduction training and structured upskilling are viewed as preventive GMP controls.
Core pharma manufacturing skills required for GMP compliance
Achieving consistent GMP performance requires balanced development across multiple skill categories:
- Technical execution skills for manufacturing and aseptic operations
- GMP decision-making and deviation handling capability
- Documentation and data integrity awareness
- Quality risk recognition and escalation judgment
- Right-first-time manufacturing discipline
Together, these skills form the foundation of a compliant and inspection-ready workforce.
How upskilling pharma manufacturing skills supports GMP inspections
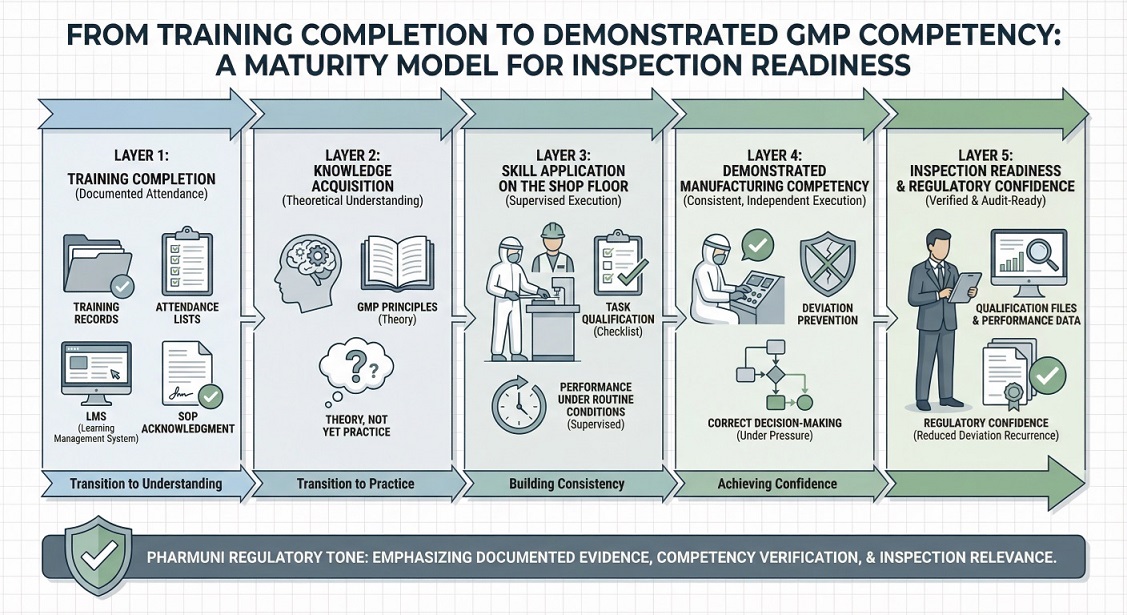
Structured upskilling enables organizations to demonstrate documented manufacturing competency during inspections. Inspectors assess not only training records but also evidence of qualification, performance consistency, and effective supervision. When workforce readiness is clearly linked to reduced deviations and controlled operations, inspection outcomes improve significantly.
When implemented systematically, upskilling for pharma manufacturing provides inspectors with tangible evidence of workforce readiness through qualification records, performance consistency, and reduced deviation recurrence.
Final Words
Manufacturing organizations that treat workforce capability as a preventive control consistently report measurable quality improvements. In several GMP remediation programs, sites that implemented structured competency frameworks achieved 20–30% reductions in deviation recurrence and noticeable improvements in right-first-time manufacturing within 12 to 18 months. These outcomes were driven by targeted skill development aligned with real shop-floor risks, not by increasing the volume of SOPs or retraining cycles.
In this context, upskilling for pharma manufacturing functions as an operational safeguard rather than a learning metric. By embedding GMP-relevant skills into daily manufacturing decisions, companies reduce inspection friction, strengthen data integrity, and demonstrate workforce readiness during regulatory inspections. Ultimately, inspection success depends less on documented training hours and more on how effectively competency is translated into consistent, compliant execution.
FAQs
In regulated production environments, workforce capability directly influences process control, deviation prevention, and right-first-time execution. When operators and supervisors lack demonstrated competency, human error increases, documentation quality declines, and inspection findings become more likely.
Completed training confirms that information was delivered, while demonstrated competency shows that personnel can correctly perform GMP-critical tasks under real operating conditions. Inspectors place greater weight on evidence of qualification, supervision, and consistent performance than on training records alone.
Inspectors review qualification files, deviation trends, error recurrence, and supervisory controls to assess whether personnel skills support controlled manufacturing. Consistent execution, low repeat deviations, and clear linkage between skills and quality outcomes are key indicators of workforce readiness.
References
1-U.S. Food and Drug Administration (FDA)
Data Integrity and Compliance With Drug CGMP – Guidance for Industry
2-European Medicines Agency (EMA)
Guideline on the principles of good manufacturing practice – Chapter 2: Personnel
3-International Council for Harmonisation (ICH)
ICH Q10: Pharmaceutical Quality System

Mahtab Shardi
Mahtab is a pharmaceutical professional with a Master’s degree in Physical Chemistry and over five years of experience in laboratory and QC roles. Mahtab contributes reliable, well-structured pharmaceutical content to Pharmuni, helping turn complex scientific topics into clear, practical insights for industry professionals and students.

Visual Inspection in Pharma (2026): What It Is, Scope, PDF Guides and SOP Essentials
Visual Inspection checks parenteral units for particles, damage, and labeling errors before release. EU GMP Annex 1 expects inspection of parenteral containers (8.30) and warns

GxP in pharma in 2026: How Inspectors Evaluate System Control
This article explains how GxP quality systems are assessed during inspections, why gaps in governance and execution lead to repeat audit findings, and how regulated

Quality Oversight In 2026: Roles in pharma industry, GMP Expectations, And Risk-Based Control
Quality Oversight keeps GMP decisions consistent, traceable, and risk-based. It connects deviation handling, CAPA, change control, and trend reviews. It also proves management engagement through


