Regulators raise the bar each year, so teams must run quality like a system. A strong QMS Implementation in pharma protects product quality, patient safety, and business continuity. In FDA’s FY2024 quality report, FDA issued 105 quality-related warning letters to human drug sites. Moreover, firms generated 260 recall events from 165 sites in FY2024.
Quality gaps also harm trust at scale. WHO estimates 1 in 10 medical products in low- and middle-income countries are substandard or falsified. Therefore, pharma leaders treat QMS as a public-health tool, not paperwork.
If you want a deeper primer, see Pharma Quality Management.
What Is QMS in the Pharmaceutical Industry?
A pharmaceutical Quality Management System (QMS) is the set of processes that control how you design, make, test, release, and improve products. It covers the full lifecycle, from tech transfer to commercial supply.
A “generic ISO QMS” is often aimed at consistency and customer satisfaction. However, a pharma QMS must also prove control under GMP expectations. It must handle validation, data integrity, batch release decisions, and regulated change. It must also connect quality to risk, process performance, and continual improvement.
In practice, a pharma QMS includes:
- Governance (quality policy, leadership review, quality metrics)
- Core GMP processes (deviations, CAPA, change control, complaints)
- Data and document control (including ALCOA+ thinking)
- Supplier and contract oversight
- Training and qualification
- Internal audits and inspection readiness routines
Regulatory Expectations for Pharma QMS
- FDA (Quality Systems approach): FDA’s guidance promotes a modern quality systems model aligned with CGMP (21 CFR 210/211) and risk management. Therefore, FDA expects a living system, not a binder.
- EU GMP Chapter 1: EU GMP Chapter 1 sets expectations for a Pharmaceutical Quality System, including management responsibility, process performance, and continual improvement. Moreover, the chapter aligns terminology with ICH Q10.
- ICH Q10 Pharmaceutical Quality System: ICH Q10 describes a comprehensive model that builds ISO concepts, includes GMP, and complements ICH Q8 and ICH Q9 (QRM). So, it gives you a global “spine” for implementation across sites and products.
Core Elements in QMS Implementation in the Pharma Industry
QMS implementation in pharma industry works best when you build strong core elements first. These elements keep GMP activities consistent, traceable, and inspection ready. Moreover, they help teams prevent repeat deviations and reduce quality risk. So, use the sections below as your practical checklist before you scale tools or expand SOP libraries.
- Quality Policy and Objectives
- Document and Data Control
- Deviation, CAPA, and Change Management
- Quality Risk Management (QRM)
- Training and Competency Management
1. Quality Policy and Objectives
Leadership must set clear quality intent, then translate it into measurable goals. Next, connect objectives to daily work.
- Define “quality” in operational terms (right-first-time, investigation cycle time, CAPA effectiveness).
- Set KPIs with thresholds and escalation rules.
- Run management review on a fixed cadence, then track actions to closure.
2. Document and Data Control
Document control prevents “shadow SOPs” and uncontrolled templates. Also, data control protects integrity and traceability.
- Build a simple hierarchy: Policy → SOP → Work Instruction → Forms.
- Control templates, versioning, and effective dates.
- Define data ownership, audit trails, and retention rules.
- Treat spreadsheets and shared drives as systems that need control.
3. Deviation, CAPA, and Change Management
These three processes form the heartbeat of GMP quality system execution. However, teams often separate them and lose the story.
- Capture deviations fast, then define impact and containment.
- Investigate root cause with evidence, not opinions.
- Design CAPAs that remove the cause, then verify effectiveness.
- Run change control with risk-based impact, validation needs, and training.
FDA links enforcement pressure to quality signals like recalls and warning letters. Therefore, strong deviation and CAPA control reduces regulatory risk.
4. Quality Risk Management (QRM)
QRM helps teams focus effort where risk lives. So, it prevents “equal effort for unequal risk.”
- Use simple tools first: risk ranking, fishbone, FMEA.
- Define risk acceptance criteria early.
- Connect QRM outputs to control strategy, validation, and ongoing monitoring.
ICH Q10 explicitly points to QRM concepts through ICH Q9 alignment.
5. Training and Competency Management
Training must prove competence, not attendance. Moreover, inspectors often ask, “How do you know people can do this?”
- Build role-based curricula per area and system.
- Use assessments, observations, and sign-offs for critical tasks.
- Track effectiveness after deviations, changes, and audit findings.
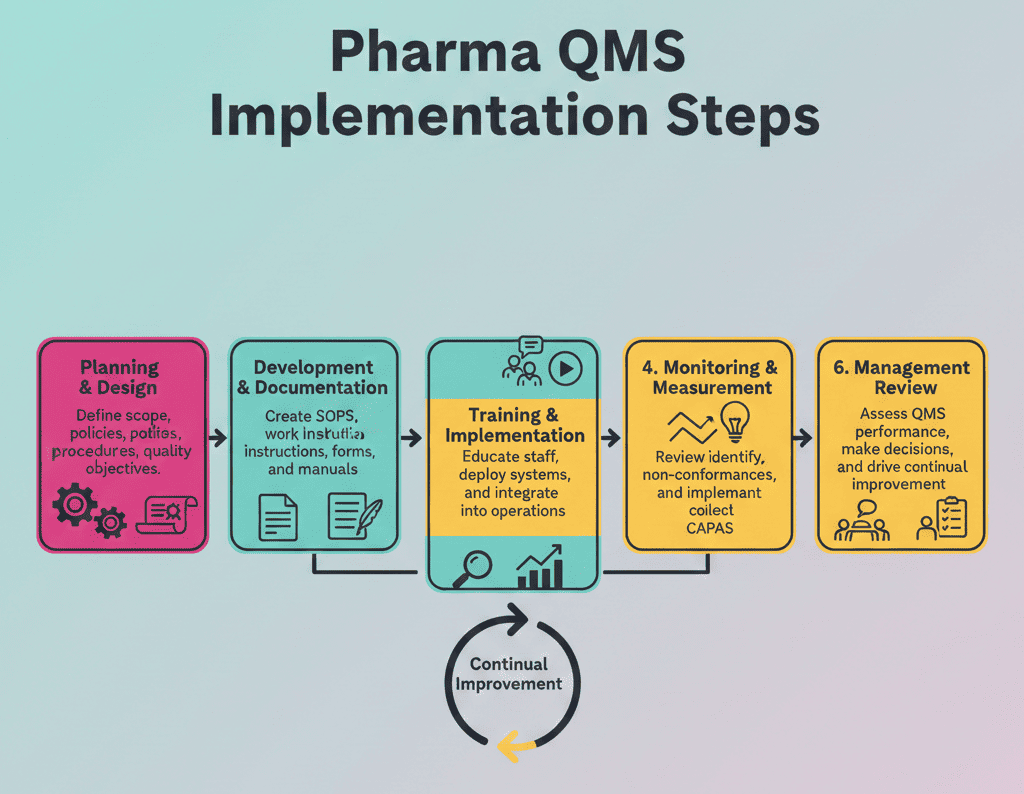
Step-by-Step QMS Implementation in Pharma Industry
- Step 1: Define scope and governance: Map your products, sites, and outsourced steps. Then assign roles for QA, QC, production, engineering, and supply chain.
- Step 2: Map processes and risks: Document the value stream from receipt to release. Next, run QRM to prioritize what you standardize first.
- Step 3: Build the core GMP processes: Implement document control, deviations, CAPA, change control, training, and supplier quality. Also, define clear interfaces between processes.
- Step 4: Enable the right tools and data flow: Select an eQMS or strengthen controlled paper workflows. Then design metrics dashboards for trends, recurring issues, and cycle times.
- Step 5: Roll out, audit, and improve: Train by role, not by department. Finally, run internal audits, management reviews, and KPI-based improvement cycles.
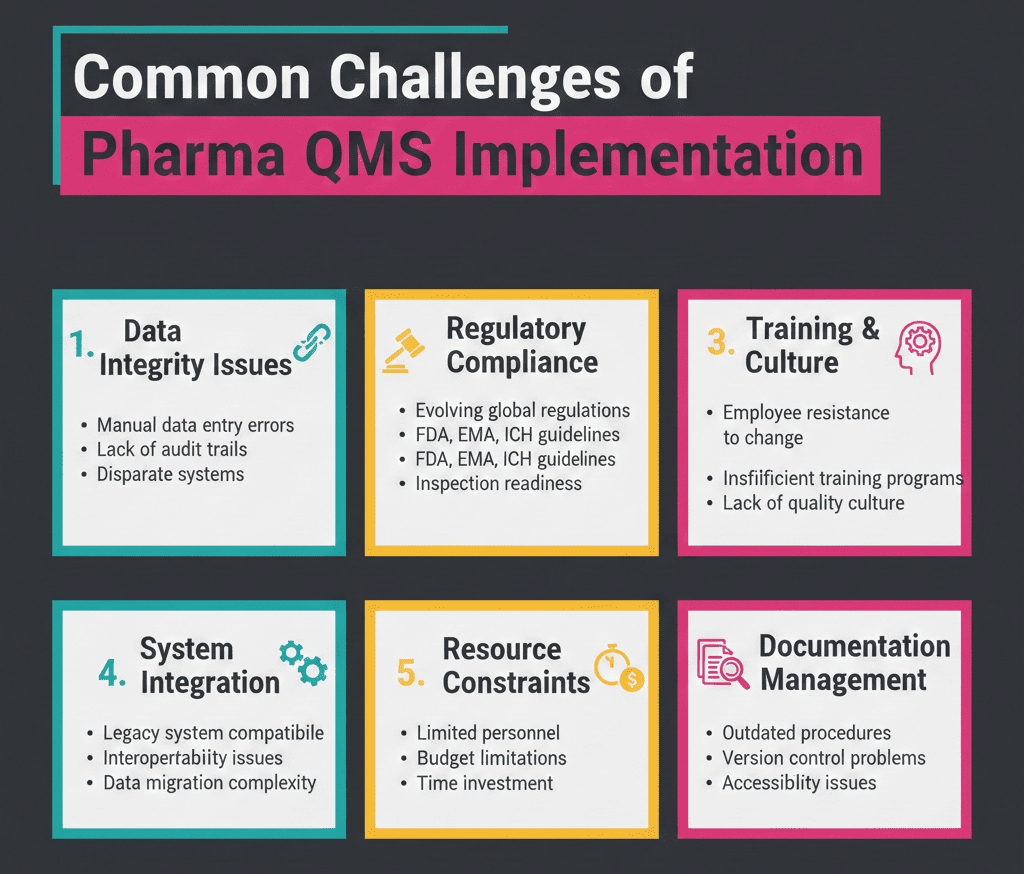
Common Challenges in Pharma QMS Implementation
Teams often hit the same obstacles. However, you can fix them with simple design choices.
- “Too many SOPs, too little clarity”: Start with high-risk processes, then expand.
- Weak investigations and “CAPA theater”: Require evidence, timelines, and effectiveness of checks.
- Siloed ownership: Define RACI for every core process. Then enforce it.
- Change overload: Use impact tiers and QRM to route changes faster.
- Supplier blind spots: Tighten qualification, quality agreements, and incoming controls.
- Data integrity drift: Standardize data review, audit trail checks, and access control routines.
- Training that proves nothing: Add task-based qualification for critical activities.
FDA’s FY2024 report shows how quality issues drive recalls and warning letters. Therefore, these “operational” problems become regulatory problems fast.
Final Words
QMS implementation in pharma industry works best when you build a system people use. So, focus on a small set of high-impact processes, then scale with risk-based discipline.
The numbers reinforce the point. In FY2024, FDA reported 260 recall events from 165 sites and 105 quality-related warning letters. Moreover, WHO still estimates 1 in 10 medicines in many markets fail quality expectations. Strong QMS design helps you reduce these outcomes through better control, faster learning, and real prevention.
FAQ:
Start by defining scope, roles, and governance for the full product lifecycle.
They check evidence of control, data integrity, and effective CAPA and change control.
Strong deviation management, CAPA effectiveness, and document control reduce repeat issues.
References:

Stephanie Männicke
Digital Marketing Especialist at Zamann Pharma Support, brings 8 years of experience in Corporate and Digital Communication. Specializing in Digital Marketing and Content Creation, Stephanie is currently focused on creating strategic content for Pharmuni's networks, especially content on topics such as recruitment, onboarding and employer branding. Outside of work, Stephanie is a mum, a crocheter and a movie fan. An avid reader and in search of expanding her knowledge, Stephanie is always looking for ways to innovate communication in the digital environment and connect people in a genuine way.

Master GxP Validation in 2026: Meaning, Key Steps, and Validated State Control
Auditors want evidence you can trace, not opinions you can explain. GxP validation links intended use, requirements, risk, and test results into one story. When

Master GMP Compliance in 2026: Meaning, Core Elements, and How to Implement
GMP compliance keeps medicines safe, consistent, and traceable across every batch. This guide explains core GMP elements, practical rollout steps, and common pitfalls. It also

History of Pharmacovigilance: From the Thalidomide Crisis (1961–2026) to GMP Oversight
Thalidomide in 1961 changed drug safety forever. Since then, pharmacovigilance has grown from crisis response to proactive risk management. Today, teams track signals, tighten reporting


