Rechallenge in pharmacovigilance supports causality assessment for an ADR. A rechallenge means the event recurs after the suspect drug restarts. Because the timing repeats, confidence increases and signals become clearer. WHO’s VigiBase holds over 40 million reports from 180+ member countries, so accurate rechallenge details matter and guide safer prescribing decisions.
In Pharmacovigilance, treat rechallenge as risk-based evidence, not a test. ICH standards need 4 minimum case elements and expedited reporting within 15 calendar days for serious, unexpected ADRs. Many rechallenges occur unintentionally after therapy resumes; capture dates, dose, outcomes, and alternatives to protect patients.
Table of Contents
Rechallenge in Pharmacovigilance Meaning
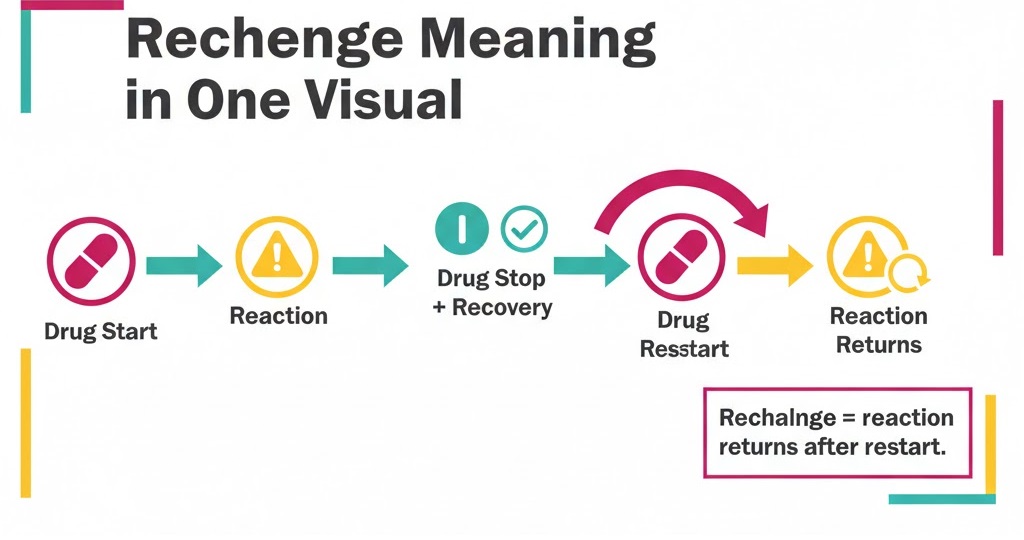
Rechallenge in Pharmacovigilance means an adverse event returns after restarting a suspect drug. It helps link timing and symptoms to the medicine, not the disease.
Therefore, teams record rechallenge because it can strengthen causality decisions. Use ICH case logic and capture the 4 minimum case elements first. Many rechallenges happen unintentionally when treatment restarts during routine care.
Write the stop and restart dates and describe the reaction clearly.
Check other causes and list concomitant medicines and infections.
Escalate high-risk cases fast to protect patients and update labels.
Rechallenge Pharmacovigilance and Related Topics
Rechallenge in pharmacovigilance means an ADR returns after the suspect drug restarts. In real cases, clinicians re-start therapy after symptoms fade. Sometimes patients self-restart medicines at home. This creates real-world rechallenge evidence for case assessment. Use the 4 minimum case elements before deeper evaluation.
Therefore, align wording with re-exposure terms and stay consistent in reports. Use “re-exposure” for any restart and “positive rechallenge” for recurrence. Then link outcomes to patient safety and risk decisions.
- Real-world context of rechallenge in ADR cases
- Rechallenge and re-exposure terminology alignment
Patient safety relevance of rechallenge
Real-World Context of Rechallenge in ADR Cases
Rechallenge occurs when a patient restarts a suspect drug and symptoms return. Clinicians may reintroduce therapy after recovery or shortage of alternatives.
Therefore, many rechallenges happen unintentionally during refills or self-medication.
ICH lists 4 minimum case elements for any report.
Refill restart
Dose increase
Brand switch
Missed counseling happens
Rechallenge and Re-Exposure Terminology Alignment
Use consistent terms in every safety case. Re-exposure means any restart after stopping the suspect drug. Therefore, label rechallenge only when the reaction returns after restart. Use “positive rechallenge” for recurrence and “negative rechallenge” for no return.
Record stop and restart dates
Keep drug names consistent
Match narrative with coded terms
Patient Safety Relevance of Rechallenge
Rechallenge can confirm a drug risk when symptoms return after restart. It helps teams act fast and prevent repeat harm. Therefore, capture key details and share them quickly.
Record restart date, dose, and reaction timing.
Alert safety teams for serious recurrences and stop re-exposure.
Rechallenge and Dechallenge Difference in Pharmacovigilance
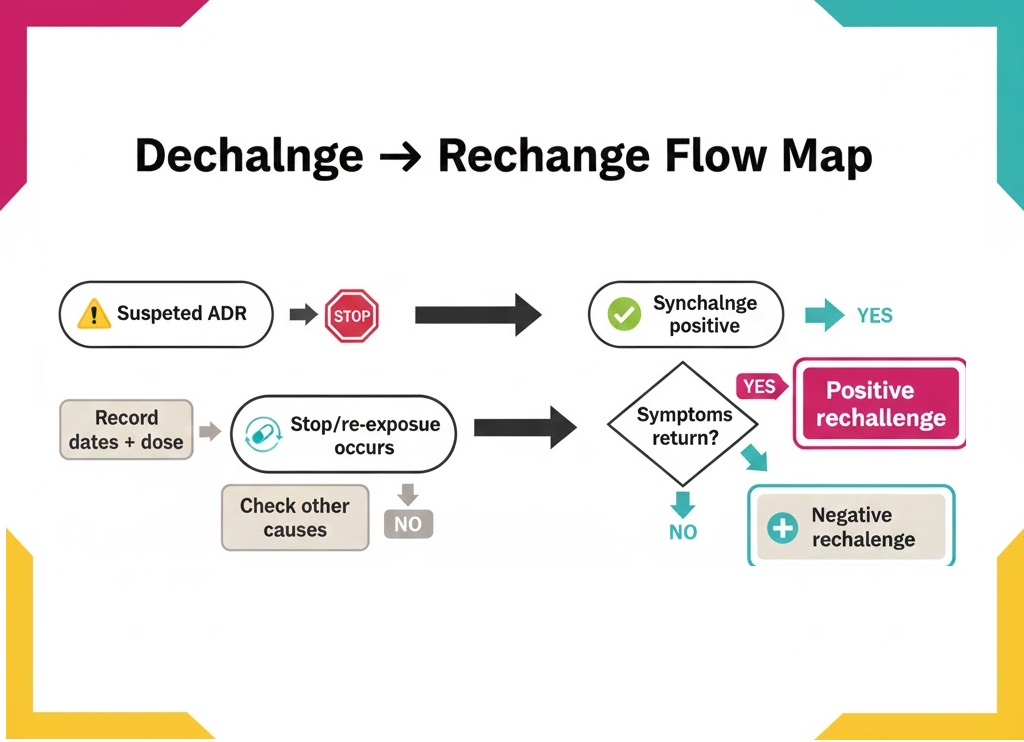
Dechallenge means symptoms improve after stopping a suspect drug in a case. Rechallenge means symptoms return after restarting the same drug. Both terms help you assess timing and plausibility in ADR reports. Dechallenge often happens during routine care and supports early risk review.
Therefore, treat dechallenge as a first signal and rechallenge as stronger evidence.
Document stop date, improvement timing, and any supportive labs.
Also record the restart details to confirm or weaken the link.
Capture restart date, dose changes, and whether symptoms recur.
Dechallenge and Rechallenge in ADR
Dechallenge means symptoms improve after stopping the suspect drug. Rechallenge means symptoms return after restarting the drug. Therefore, document both actions clearly to support causality and safety decisions.
Record stop date and improvement timing
Record restart date and recurrence timing
Note dose changes and formulation switches
Check other causes and list concomitant medicines
Timing, Dose, and Response Criteria
ICH expects expedited reporting within 15 calendar days for serious, unexpected ADRs. Accurate timing, dose, and response notes help you meet that clock. Use exact times when you can, especially for rapid reactions.
Therefore, record key criteria as soon as you confirm re-exposure. Keep the narrative and coded fields aligned.
Capture start, stop, and restart dates
Log dose, route, and frequency changes
Note time-to-onset and time-to-recovery
Describe recurrence, severity, and objective evidence
Final Words
Rechallenge in pharmacovigilance means an adverse reaction returns after re-starting a suspect drug. It strengthens causality because the event repeats with renewed exposure. WHO’s global database VigiBase holds over 40 million reports from 180+ programme members. It began in 1968 with 10 founding members, so clear rechallenge notes improve signal detection and patient protection.
This guide covered meaning, ICSR placement, and validity checks. Build your Pharmacovigilance Career Path by practicing clean documentation and risk thinking.
Start here:
(1) record stop/restart dates and dose,
(2) code outcomes consistently,
(3) escalate serious recurrences fast.
FAQs
Rechallenge means the reaction returns after you restart the suspect drug. It can support causality when timing and symptoms repeat. ICH still needs a valid ICSR first.
No. Clinicians may restart therapy during routine care. Patients may also restart after refills. So you must document it as risk-based evidence, not a “test.”
Good rechallenge data improves signal evaluation across large datasets. VigiBase holds 40+ million reports from 180+ WHO PIDM members. Your case quality supports safer use decisions.
References

Ershad Moradi, a Content Marketing Specialist at Zamann Pharma Support, brings 6 years of experience in the pharmaceutical industry. Specializing in pharmaceutical and medical technologies, Ershad is currently focused on expanding his knowledge in marketing and improving communication in the field. Outside of work, Ershad enjoys reading and attending industry related networks to stay up-to-date on the latest advancements. With a passion for continuous learning and growth, Ershad is always looking for new opportunities to enhance his skills and contribute to pharmaceutical industry. Connect with Ershad on Facebook for more information.

Master GxP Validation in 2026: Meaning, Key Steps, and Validated State Control
Auditors want evidence you can trace, not opinions you can explain. GxP validation links intended use, requirements, risk, and test results into one story. When you control changes and review performance, you keep the system inspection-ready every day on time.

Master GMP Compliance in 2026: Meaning, Core Elements, and How to Implement
GMP compliance keeps medicines safe, consistent, and traceable across every batch. This guide explains core GMP elements, practical rollout steps, and common pitfalls. It also shows how to strengthen training, documentation, data integrity, and audit readiness.

History of Pharmacovigilance: From the Thalidomide Crisis (1961–2026) to GMP Oversight
Thalidomide in 1961 changed drug safety forever. Since then, pharmacovigilance has grown from crisis response to proactive risk management. Today, teams track signals, tighten reporting rules, and connect safety data to quality systems. As a result, PV now links directly to GMP oversight, audits, and data integrity.


