Pharma companies handle millions of safety data points each year. Global Pharmacovigilance activities already form a market of over USD 10 billion in 2025 and may exceed USD 22 billion by 2034. At the same time, the AI in pharmacovigilance market itself was valued at about USD 600 million in 2024 and could reach nearly USD 2 billion by 2034, with an impressive CAGR above 20%.
Pharmacovigilance teams must manage case intake, coding, follow-up, reporting, and signal detection. However, data now arrives from many channels: EHR systems, social media, patient apps, and global safety databases. Traditional manual workflows struggle with this scale.
This mix of high potential and low adoption makes AI in PV a strategic topic for 2025. The rest of this guide explains what changes, how the tools work, and what it means for careers and compliance.
What Is the Impact of AI in Pharmacovigilance?
AI in PV augments, not replaces, drug safety experts. Instead of reading every narrative from scratch, teams can let AI pre-structure data, highlight patterns, and flag possible risks. Humans then focus on judgement, medical reasoning, and regulatory decisions.
First, AI helps with volume. Machine learning systems can scan large public databases such as FAERS, VigiBase, and EVDAS, alongside internal safety systems, much faster than traditional methods. Second, AI improves consistency in coding and triage, which supports reliable signal detection. Automated workflows lower operational costs and free resources for complex benefit–risk work.
However, the impact is not only about speed. AI can also:
- Prioritize cases with high clinical risk.
- Detect subtle patterns across products, indications, and regions.
- Support earlier recognition of safety signals from real-world data.
Regulators and expert groups such as CIOMS see clear potential but also highlight risks around bias, explainability, and data quality.
Therefore, Pharmacovigilance automatation must operate with strong governance, transparent algorithms, and continuous human oversight.
Core Pharmacovigilance Processes Before AI in Pharmacovigilance
To understand the change, it helps to revisit core PV processes.
Traditionally, safety teams:
- Receive adverse event reports from healthcare professionals, patients, partners, and regulators.
- Validate and code cases using MedDRA and other dictionaries.
- Enter and clean data in a safety database.
- Prepare expedited reports and periodic safety updates.
- Run signal detection and risk evaluation activities.
These tasks rely on careful reading and manual entry. Many steps repeat the same actions across cases. Moreover, growing data volumes from clinical trials, post-marketing use, and patient support programs increase workload every year.
Without machine learning in pharmacovigilance, teams often face backlogs, overtime, and delayed signal review. Consequently, organizations search for smarter tools that automate low-value steps while staying compliant.
AI Applications in PV Processes
Now, AI in PV reshapes each step of the workflow.
- Automated case intake: NLP engines read emails, call center transcripts, forms, and social posts. They extract key data fields such as suspect drug, reaction, patient age, and seriousness.
- Smart coding and normalization: AI recommends MedDRA terms, drug codes, and product names, which safety specialists then confirm.
- Case triage and prioritization: Machine learning models rank cases by clinical risk, missing data, or regulatory due dates.
- Signal detection AI: Advanced analytics scan many data sources and spot unusual patterns or disproportional signals earlier.
- Safety trend analysis: Predictive models monitor changes over time and support risk-based surveillance.
As a result, organizations that deploy pharmacovigilance automatation often report faster cycle times, fewer manual errors, and more focused on clinical review. However, they also need robust validation, audit trails, and change control to satisfy regulators.
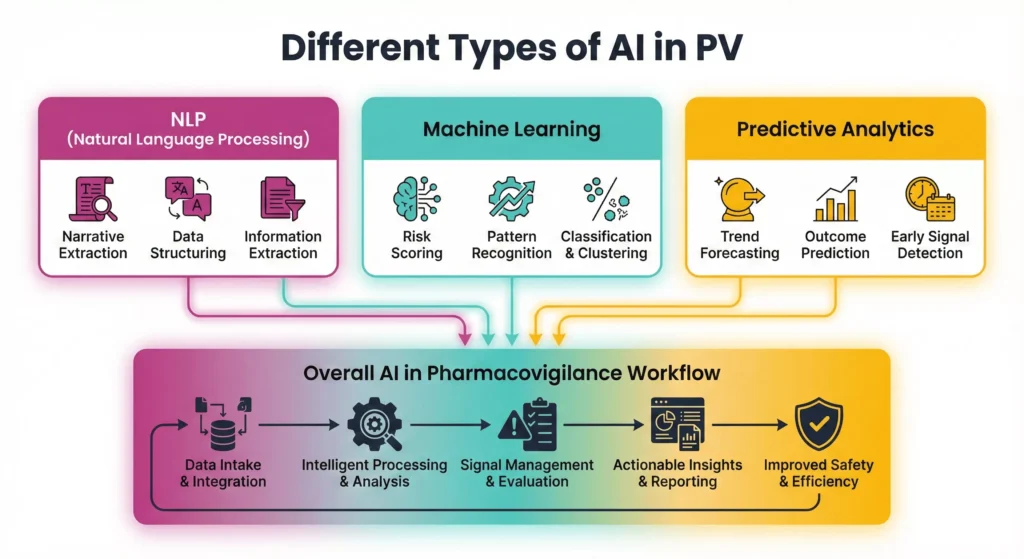
AI Technologies Used in Pharmacovigilance
Different technologies combine modern AI tools in pharmacovigilance platforms. Vendors usually integrate several layers:
- Natural language processing (NLP) for unstructured text.
- Machine learning models for classification, clustering, and prioritization.
- Predictive analytics and risk-based monitoring engines.
- Sometimes robotic process automation (RPA) to orchestrate tasks across systems.
Together, these tools connect with safety databases, CRM systems, medical information platforms, and regulatory gateways. Strong data governance keeps this landscape secure and compliant.
Natural Language Processing (NLP)
Natural Language Processing turns messy safety narratives into structured, usable data. Instead of reading every email, call note, or report from scratch, teams let NLP engines scan text, pick out the most important details, and feed them into AI in pharmacovigilance workflows. This step saves time, reduces typing errors, and makes later signal detection more reliable.
NLP in PV typically helps to:
- Extract key entities such as drug names, adverse events, doses, and patient details.
- Classify documents by type (spontaneous report, literature case, medical inquiry) and case validity.
- Suggest MedDRA terms and product codes for faster, more consistent coding.
- Flag missing or conflicting information so case processors can request targeted follow-up.
Machine Learning
Machine learning in PV learns from historical cases and decisions. Instead of fixed rules, models adjust as they see more data.
Common uses include:
- Predicting which cases need faster medical review.
- Classifying seriousness or expected patterns.
- Grouping similar cases for signal assessment.
Moreover, ML models support risk-based monitoring and targeted follow-up strategies. Safety leaders gain dashboards that highlight products, patient groups, or regions that deserve extra focus.
Regulators, however, ask for transparency. Therefore, organizations increasingly prefer explainable models that show which features drive each prediction.
Predictive Analytics
Looking beyond today’s cases, predictive analytics estimate what may happen next. Models combine spontaneous reports with clinical data, prescription trends, and even social listening signals.
This allows safety teams to:
- Spot emerging risk patterns earlier in the product lifecycle.
- Test “what-if” scenarios for label changes or risk minimization plans.
- Plan resources for periods of high reporting volume.
In addition, predictive analytics supports risk-based monitoring and real-time safety surveillance, two key ambitions for modern PV systems. For example, dashboards can highlight products where risk indicators rise above a configured threshold and trigger deeper review.
Typical applications and types of predictive analytics in PV include:
- Descriptive analytics: Summarizes past safety data to show patterns by product, region, or patient group.
- Diagnostic analytics: Explores why certain safety issues increased, using drill-down views and correlative analysis.
- Predictive analytics: Forecasts potential safety hotspots, reporting surges, or signal emergence before they fully appear.
- Prescriptive analytics: Recommends next best actions such as extra monitoring, label changes, or targeted risk minimisation.

Future Trends in Artificial Intelligence and PV
As adoption grows, AI in PV will move far beyond single-use tools and isolated pilots. Safety teams will connect AI engines across the full PV lifecycle, from case intake to risk management. At the same time, regulators, vendors, and pharma companies will shape clearer rules, better validation methods, and new skill requirements. The trends below highlight how artificial intelligence and PV will likely evolve over the next few years and what this means for drug safety professionals.
- Generative and agentic AI for end-to-end workflows: Multi-step “agents” will draft narratives, pre-fill regulatory reports, and orchestrate PV tasks under human oversight.
- Deeper integration with real-world data sources: AI in pharmacovigilance will pull signals from EHRs, claims data, patient apps, and social media to strengthen real-time safety surveillance.
- Explainable and auditable AI models: Transparent algorithms and clear audit trails will become standard to meet regulatory expectations and support GxP inspections.
- Risk-based and predictive safety monitoring: Advanced analytics will shift PV from reactive case review to proactive, risk-based monitoring with early warning dashboards.
- New hybrid skill sets and PV career paths: Drug safety roles will increasingly combine pharmacovigilance expertise with data literacy, AI tool knowledge, and digital process design.
Final Words
Across this article, AI in pharmacovigilance emerges as a practical ally for modern drug safety teams. Automation now supports case intake, coding, triage, and signal detection, while NLP and predictive analytics uncover patterns that manual review might miss. However, human experts still lead every critical decision. Medical reviewers validate signals, interpret complex cases, and explain benefit–risk decisions to regulators. AI simply accelerates the heavy data work so specialists can focus on scientific and regulatory judgement.
For current and future professionals, this shift creates strong career opportunities. Drug safety roles now benefit from a blend of PV knowledge, regulatory understanding, and confidence with AI-enhanced tools. People who invest in these skills position themselves for high-impact roles in pharma, biotechs, CROs, and regulatory environments. Anyone who wants a clear roadmap can follow a structured Pharmacovigilance Career Path that covers core safety science, global regulations, and digital competencies. With this foundation, AI becomes a powerful amplifier of expertise and helps build safer therapies and smarter global safety systems.
FAQ:
AI in PV means using tools like machine learning and natural language processing to support drug safety work. These tools help collect, structure, and analyse safety data so teams detect risks faster and make better benefit–risk decisions.
First, AI reads unstructured sources such as emails, call transcripts, and medical notes. Then, it extracts key details like drug name, reaction, and seriousness. This case processing automation reduces typing errors, speeds data entry, and keeps reports more consistent across large volumes.
Several technologies now support pharmacovigilance automation:
- NLP for extracting information from narratives and free text
- Machine learning for case triage, classification, and signal detection
- Predictive safety analytics for trend forecasting and risk-based monitoring
Together, these tools boost pharmacovigilance workflow efficiency and support real-time safety surveillance.
References:

Stephanie Männicke
Digital Marketing Especialist at Zamann Pharma Support, brings 8 years of experience in Corporate and Digital Communication. Specializing in Digital Marketing and Content Creation, Stephanie is currently focused on creating strategic content for Pharmuni's networks, especially content on topics such as recruitment, onboarding and employer branding. Outside of work, Stephanie is a mum, a crocheter and a movie fan. An avid reader and in search of expanding her knowledge, Stephanie is always looking for ways to innovate communication in the digital environment and connect people in a genuine way.

Revalidation In Pharma (2026 guide): Meaning, Triggers, Frequency, And Requalification Differences
Revalidation protects product quality and business continuity by linking GMP decisions to risk, evidence, and context. This article helps teams decide when to act, what

GMP Regulations in 2026: How Inspectors Assess Compliance and Control Systems
This article explains how pharmaceutical regulatory requirements shape inspection decisions, why GMP compliance gaps persist across manufacturing sites, and which operational controls, documentation practices, and

WHO GMP in 2026: Inspection Readiness and Compliance Expectations
This article explains how global pharmaceutical GMP standards are applied during inspections, why operational gaps persist despite formal compliance, and how quality systems, contamination control,


