Today, medicines follow strict rules called Good Manufacturing Practices, but in the early 1900s weak laws allowed unsafe products. Disasters like the 1906 misbranding cases and the deadly 1937 sulfanilamide incident pushed regulators to demand real safety proof and controls, creating the foundation for the modern history of Good Manufacturing Practices.
In 1938, the Federal Food, Drug, and Cosmetic Act gave the FDA stronger powers. Later, the 1962 Kefauver–Harris Amendments and the first FDA GMP regulations in 1963 turned quality expectations into enforceable law. Meanwhile, WHO and the EU built their own GMP frameworks. Together, these events created today’s global GMP standards that protect patients worldwide.
Why GMP Was Created: Early Problems in Drug Manufacturing
Before modern regulations, drug manufacturing often looked chaotic and unsafe. Factories lack standardized procedures. Labels made big promises but rarely showed full risks or ingredients.
Contaminations
Manufacturers mixed drugs in shared, poorly cleaned vessels. As a result, products often contain unknown impurities, microbes, or foreign particles. Some batches harmed patients even when the active ingredient itself was safe.
Mislabeling
Companies used bold marketing and weak science. They sold tonics as “cures” without proof. Therefore, patients could not know what they received or whether the product contained dangerous solvents, heavy metals, or narcotics.
Lack of production controls
Workers followed personal habits instead of written procedures. No one tracked critical parameters such as temperature, mixing times, or filtration steps. Consequently, one batch might work, while the next failed silently.
Variability in drug quality
Because controls were weak, potency varied strongly from batch to batch. Sometimes patients received too little drug and stayed ill. Sometimes they received too much and faced serious side effects. These failures slowly built the case for strict, enforceable manufacturing rules.
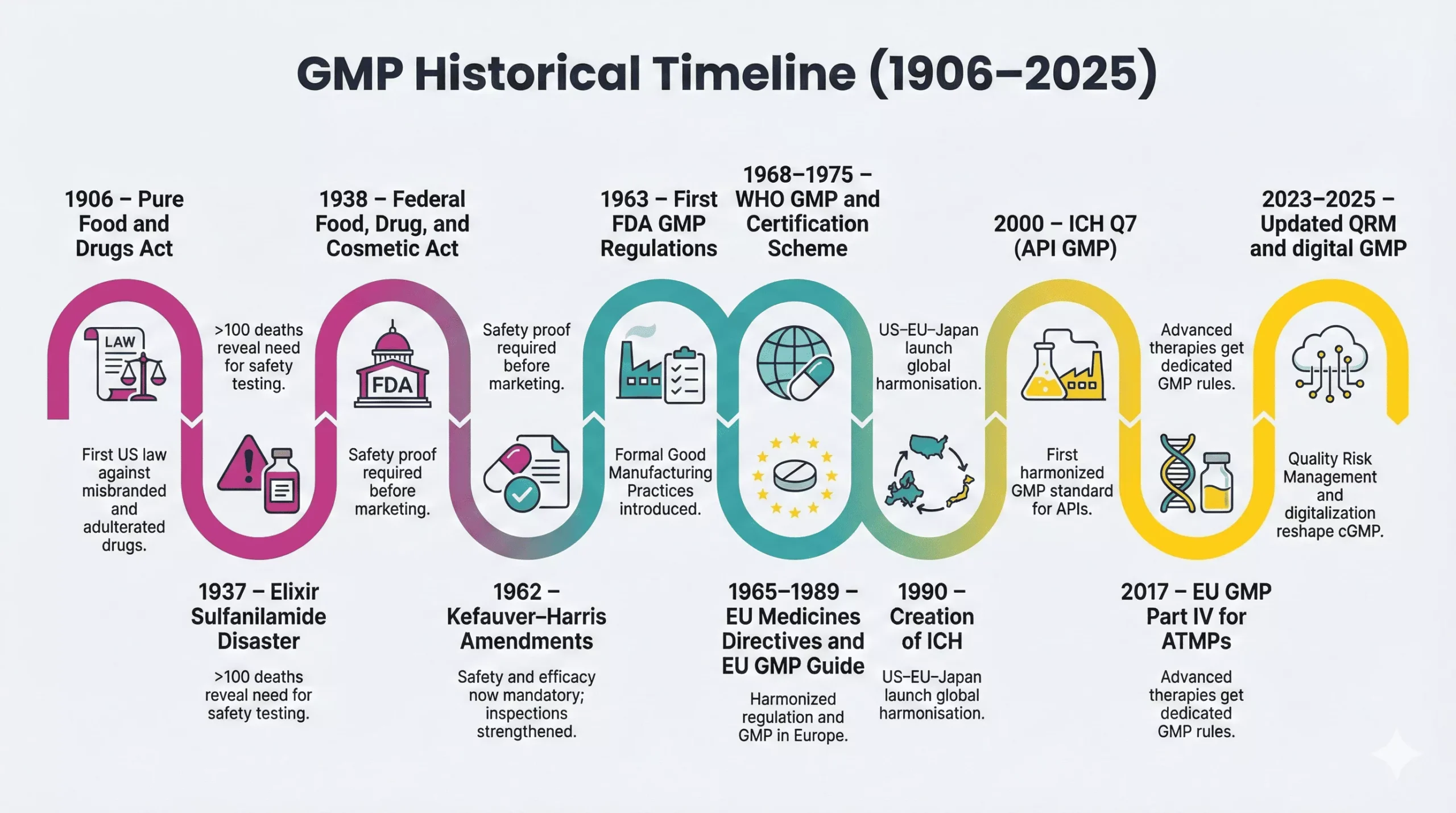
Early Foundations of GMP (1900–1940)
The history of Good Manufacturing Practices starts with basic consumer protection laws. Between 1900 and 1940, three milestones shaped the first layer of regulation.
1906: Pure Food and Drugs Act
In 1906, the Pure Food and Drugs Act banned the sale of misbranded or adulterated food and drugs in interstate commerce. This law created a legal foundation for federal oversight and later inspired the formation of the FDA as a dedicated guardian of public health.
The Act still had limits. It focused on labeling and obvious adulteration. It did not require pre-market proof of safety. However, it sent a clear message: manufacturers could no longer sell anything they wanted without consequences.
1937: Sulfanilamide Tragedy
In 1937, a company formulated a liquid version of sulfanilamide using diethylene glycol, a toxic solvent. More than 100 adults and children died across the United States. No law required toxicity testing of the new elixir, so the company broke no specific regulation at that time.
This tragedy shocked the public and politicians. It showed that safety could not rely on trust or marketing claims. Instead, regulators needed power to demand testing, documentation, and control over how medicines reached the market.
1938: Federal Food, Drug, and Cosmetic Act
In response, Congress passed the Federal Food, Drug, and Cosmetic Act in 1938. The law required proof of safety before new drugs entered the market and expanded FDA’s authority over manufacturing, labeling, and inspections.
From this moment, manufacturers had to treat safety as a legal obligation, not a voluntary promise. The Act did not yet use the term “Good Manufacturing Practices,” but it created the regulatory backbone that would later support formal GMP rules.
Birth of Modern GMP Standards (1940–1970)
The thalidomide disaster in Europe and Canada, with thousands of birth defects, triggered a major shift in U.S. law. The 1962 Kefauver–Harris Amendments required manufacturers to prove both safety and efficacy before marketing drugs and strengthened FDA control over manufacturing and advertising.
Importantly, the Amendments authorized the FDA to set Good Manufacturing Practice requirements and to inspect plants on a regular basis. This link between product approval and manufacturing quality laid down a key stone in the GMP regulatory history.
After World War II, pharmaceutical science advanced quickly. New antibiotics, vaccines, and chronic disease treatments moved from labs into mass production. However, this progress brought fresh risks and complexity.
1950s–1960s: Rise of Mass Manufacturing
During the 1950s and 1960s, companies built large production plants and supplied medicines to global markets. Doses became more potent, processes became more intricate, and supply chains grew longer.
Therefore, small errors in one facility could harm thousands of patients worldwide. Governments saw that traditional inspection approaches no longer worked. They needed structured, preventive rules that covered every step of manufacturing, not only final product testing.
1962: Kefauver–Harris Amendments
The thalidomide disaster in Europe and Canada, with thousands of birth defects, triggered a major shift in U.S. law. The 1962 Kefauver–Harris Amendments required manufacturers to prove both safety and efficacy before marketing drugs and strengthened FDA control over manufacturing and advertising.
Importantly, the Amendments authorized the FDA to set Good Manufacturing Practice requirements and to inspect plants on a regular basis. This link between product approval and manufacturing quality laid down a key stone in the GMP regulatory history.
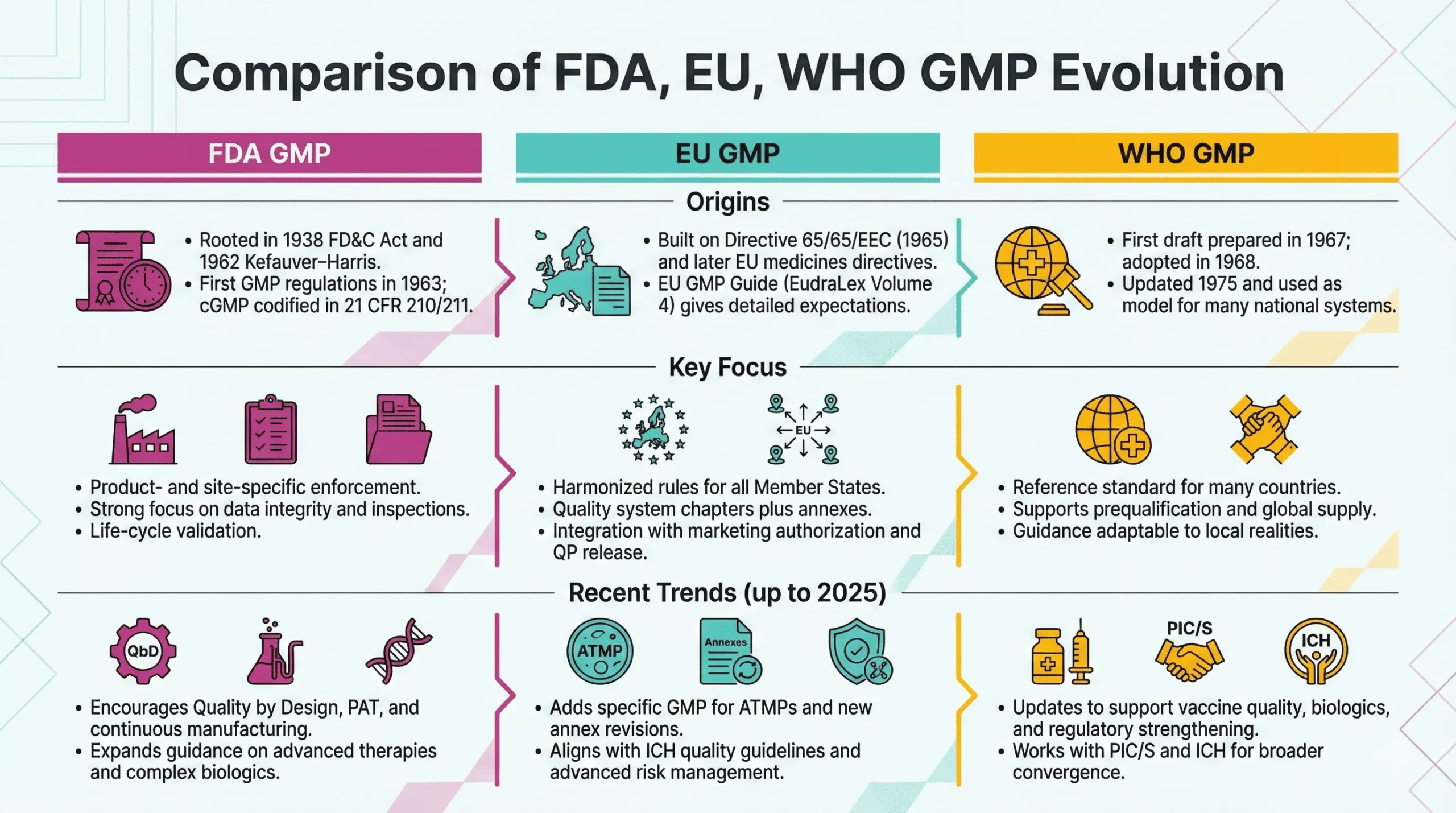
1963: First FDA GMP Regulations
In 1963, FDA published the first GMP regulations for drugs under Section 501(a)(2)(B) of the FD&C Act. These rules defined baseline expectations for facilities, equipment, records, and quality control.
Later, in 1978, FDA updated and codified “current” Good Manufacturing Practice (cGMP) in 21 CFR Parts 210 and 211, which still form the U.S. GMP backbone today. From this point, modern GMP moved from guidance to enforceable law with direct impact on approvals, inspections, and enforcement actions.
Global Expansion of GMP Standards
As pharmaceutical trade globalized, individual national systems no longer sufficed. Countries needed shared expectations so they could trust each other’s products and inspections.
WHO GMP (1968, updated 1975)
Recognizing this need, the World Health Organization prepared its first draft text on GMP in 1967, adopted the initial version in 1968, and integrated GMP into its Certification Scheme in 1969. In 1975, WHO adopted revised versions of both the scheme and the GMP text, giving regulators a global reference for manufacturing quality.
WHO GMP guidelines helped many countries build or modernize their own regulations. They also supported safer international trade in medicines, especially in low- and middle-income markets.
EU GMP Introduction
In Europe, the thalidomide crisis sparked harmonized medicines legislation. Council Directive 65/65/EEC in 1965 required prior to marketing authorization and set common principles for drug safety, quality, and efficacy. Later directives in 1975 further aligned testing standards and authorization procedures.
Over time, the European Commission consolidated GMP guidance into EudraLex Volume 4, the EU GMP Guidelines for human and veterinary medicines. These guidelines interpret legal principles and include annexes for sterile products, biologicals, and advanced therapies, creating a detailed regional framework.
Increasing Global Harmonization
From the late 20th century onward, harmonization became a central theme in the evolution of GMP. Several initiatives reinforced this trend:
- PIC/S (Pharmaceutical Inspection Convention and Scheme): The Pharmaceutical Inspection Convention started in 1970, focusing on mutual recognition of inspections. In 1995, authorities created PIC/S to expand this cooperation and align inspector training and GMP standards across many countries.
- ICH (International Council for Harmonization): In 1990, regulators and industry from the US, EU, and Japan launched ICH to harmonize technical requirements for drug registration. ICH later expanded globally and issued key quality guidelines.
- ICH Q7 and modern quality guidelines: In 2000, ICH released Q7, the first harmonized GMP guideline for active pharmaceutical ingredients (APIs). Later, Q8, Q9, and Q10 promoted Quality by Design and formal Quality Risk Management, pushing companies toward science- and risk-based GMP systems.
Today, regulators keep refining GMP for new technologies such as advanced therapy medicinal products, reflected for example in the EU’s dedicated GMP Part IV in 2017.
Key Milestones That Shaped the History of GMP
Over more than a century, three forces have kept reshaping the history of Good Manufacturing Practices: major safety events, technology shifts, and regulatory harmonization.
Major Drug Safety Events
- Public tragedies as catalysts: Events like the Sulfanilamide disaster and thalidomide crisis showed that weak production controls can destroy lives and trust.
- Ongoing enforcement cases: Therefore, every major GMP failure today—contaminated injectables, data integrity scandals, or sterile breaches—usually leads to new guidance, warning letters, and sometimes updated regulations.
Technology Advancements
- From compounding to continuous manufacturing: Early GMP dealt with simple batch processes. Now, companies use automation, continuous manufacturing, real-time analytics, and digital batch records. Consequently, modern GMP emphasizes data integrity, computerized systems, and lifecycle validation.
- Shift toward risk- and science-based control: Tools-like Quality by Design and Quality Risk Management help teams focus on controls where they matter most. Therefore, GMP no longer stops checklists; it demands deep process understanding.
Regulatory Harmonization
- Converging standards: WHO GMP, EU GMP, FDA cGMP, and ICH guidelines now overlap strongly. Differences remain, but core expectations around quality systems, documentation, validation, and change control look very similar.
- Mutual recognition and global supply chains: Arrangements under PIC/S and mutual recognition agreements reduce duplicate inspections and support smoother supply across borders. At the same time, they raise the bar for compliance because one inspection outcome can affect many markets.
Final Words
The history of Good Manufacturing Practices shows how unsafe products, weak laws, and tragedies pushed society to demand protection. Governments and industry turned those lessons into structured GMP systems that control facilities, equipment, processes, and data so every batch of medicine meets strict safety and quality standards.
The story keeps evolving. New technologies, advanced therapies, and digital tools reshape factories and regulators’ expectations, while global harmonization and risk management strengthen GMP further. For professionals in QA, production, regulatory affairs, and pharmacovigilance, this history turns GMP from a checklist into a truly patient-focused quality culture.
FAQ:
The history of GMP starts in 1906 with early US food and drug laws. Then, tragedies like the 1937 sulfanilamide disaster and the thalidomide crisis pushed regulators to act. Over time, FDA, WHO, and the EU created structured GMP rules to control every step of drug manufacturing.
Regulators created GMP rules because unsafe, contaminated, and mislabeled medicines harmed patients. Early factories lacked process controls, documentation, and testing. Therefore, new laws required proof of safety, standardized procedures, and inspections so manufacturers could not rely on guesswork or marketing claims.
Key milestones include the 1906 Pure Food and Drugs Act, the 1938 Federal Food, Drug, and Cosmetic Act, and the 1962 Kefauver–Harris Amendments. Then, the first FDA GMP regulations in 1963, WHO GMP in 1968, and EU GMP guidelines further shaped global standards. Later, ICH and PIC/S drove international harmonization.
WHO issued global GMP guidance that many countries used as a model for national laws. At the same time, the EU created the EU GMP Guide and linked it to centralized marketing authorization. Together, they pushed a more consistent, harmonized approach to GMP across regions.
This history shows that every major GMP rule exists because patients once suffered from poor quality or weak oversight. When professionals understand this context, they see GMP as a patient protection tool, not just paperwork. As a result, teams apply regulations more thoughtfully and build stronger quality systems.
References:

Stephanie Männicke
Digital Marketing Especialist at Zamann Pharma Support, brings 8 years of experience in Corporate and Digital Communication. Specializing in Digital Marketing and Content Creation, Stephanie is currently focused on creating strategic content for Pharmuni's networks, especially content on topics such as recruitment, onboarding and employer branding. Outside of work, Stephanie is a mum, a crocheter and a movie fan. An avid reader and in search of expanding her knowledge, Stephanie is always looking for ways to innovate communication in the digital environment and connect people in a genuine way.

Revalidation In Pharma (2026 guide): Meaning, Triggers, Frequency, And Requalification Differences
Revalidation protects product quality and business continuity by linking GMP decisions to risk, evidence, and context. This article helps teams decide when to act, what

GMP Regulations in 2026: How Inspectors Assess Compliance and Control Systems
This article explains how pharmaceutical regulatory requirements shape inspection decisions, why GMP compliance gaps persist across manufacturing sites, and which operational controls, documentation practices, and

WHO GMP in 2026: Inspection Readiness and Compliance Expectations
This article explains how global pharmaceutical GMP standards are applied during inspections, why operational gaps persist despite formal compliance, and how quality systems, contamination control,


