Pharma engineering courses train professionals to design, validate, and improve safe manufacturing systems. ISPE reports that over 70% of pharma facilities now prioritize modernization and digitalization, which shows how crucial engineering roles are in GMP production. As a result, employers increasingly seek people who understand both process design and regulatory expectations.
Today’s pharma courses must therefore blend engineering, quality, and data skills. According to ISPE surveys, more than 60% of companies struggle to hire talent with strong cross-functional expertise. Ppharma engineering degree close this gap and prepare learners for automated, data-driven plants.
Table of Contents
What Is Pharma Engineering?
Pharmaceutical engineering combines pharmacy knowledge with engineering methods to create safe medicines. It studies how ingredients move and transform in automated lines. Engineers design cleanrooms, select equipment, and plan workflows that protect patients. Therefore they link science, technology, and regulations, while over 60% of pharma investment now targets facilities and technology.
Pharmaceutical engineering courses turn these ideas into practical factory skills. Students learn to plan processes, scale production, and reduce errors. They translate guidelines into concrete checks and use automation, sensors, and data tools for faster decisions. With job growth around 5–7% in many regions, these skills support strong long-term careers.
Drug manufacturing fundamentals and unit operations
Process design, mapping, and optimization
Equipment qualification, commissioning, and validation
Scale-up from lab to pilot and full plant
Key Pharma Engineering Courses
Key pharma engineering degrees usually fall into three main blocks that link science, technology, and GMP. The first block covers chemical and bioprocess engineering, including mass and heat transfer, unit operations, and fermentation. Biotechnology engineering adds genetic engineering and advanced therapies, such as monoclonal antibodies and cell or gene products.
The second block focuses on industrial production: pharma manufacturing for solid, liquid, and sterile dosage forms, plus scale-up. Industrial pharmacy adds formulation, stability, packaging, and technology transfer, and process engineers remain highly in demand. The final block centers on quality and regulation, with GMP training on FDA 21 CFR 210–211 and ICH Q8–Q10. Quality systems topics—QMS, change control, risk management, batch review—help reduce the GMP warning-letter risk that the FDA reports every year.

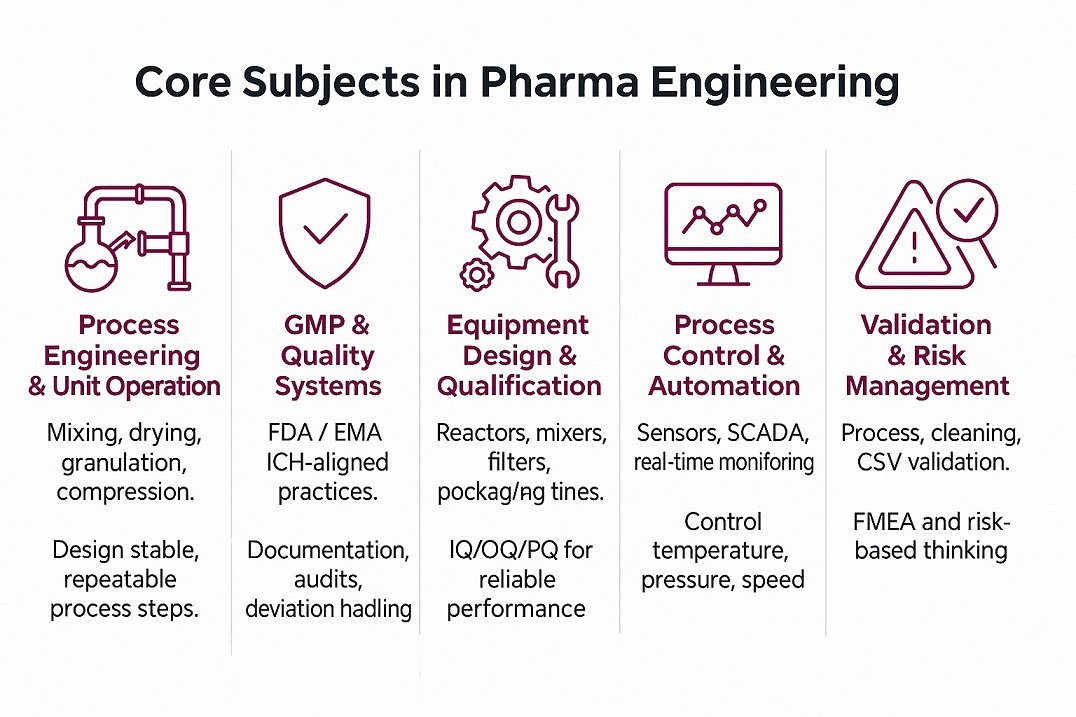
Core Subjects in Pharma Engineering
Core subjects in pharma engineering give you tools for real plant problems. You learn how materials move, mix, heat, and transform safely. Process Engineering and Unit Operations link lab ideas to full-scale lines. GMP and Quality Systems add rules that protect patients and products, so you see how science and compliance work together.
Equipment Design and Qualification help you choose and prove reliable machines. Process Control and Automation let you track data and adjust fast. Validation and Risk Management show that processes work and stay under control. Together, these subjects turn theory into a job-ready skill set for factory and QA roles.
Comparison by focus
Process Engineering and Unit Operations: flow, mixing, and physical transformation steps.
GMP and Quality Systems: rules, documentation, and overall plant governance.
Validation and Risk Management: proof of control and structured risk decisions.
Comparison by daily impact
Equipment Design and Qualification: you select, qualify, and maintain reliable machines.
Process Control and Automation: you monitor trends and adjust parameters in real time.
GMP and Quality Systems: you log events and solve issues with data.
Types of Pharma Engineering Courses (Diploma, UG, PG & Certifications)
Pharma engineering offers several study paths with different depth, time, and cost. Diplomas give focused foundations in two to three years. BTech or MTech programs go deeper and support advanced technical roles. Short courses and distance programs add flexible options for busy professionals.
Each option suits different goals and life situations. Therefore, compare duration, cost, and career impact before you choose. Diplomas help school leavers enter industry sooner. Additionally, advanced degrees and flexible programs support growth or a career switch into pharma engineering.
By duration and depth
Diploma – medium depth, 2–3 years
BTech/MTech – highest depth, 4–6 years
Distance programs – variable depth, flexible pace
Short-term certifications – focused depth, weeks or months
By who it suits
Diploma – recent school graduates entering industry
BTech/MTech – future leaders and specialists
Distance programs – working professionals needing flexibility
Short-term certifications – experts updating niche skills
Career Opportunities After Pharma Engineering
Pharma engineering opens many career paths in global medicine production. Graduates join roles in manufacturing, process design, and quality. Moreover, they support safe scale-up from lab concepts to reliable plant output worldwide.
You can work as a process engineer, validation engineer, or production supervisor. Additionally, many move into GMP quality roles, such as QA or QC specialist. These positions handle deviations, documentation, and continuous improvement projects on the shop floor.
Later, experience unlocks higher-level responsibilities and leadership. Some professionals lead new facility projects or digitalization programs. Others shift toward regulatory support, technical sales, or consulting for pharma and biotech clients.
Salary Trends in 2026
Pharma engineers earn different salaries worldwide. Junior engineers often make $30,000–$60,000 yearly. Therefore, senior experts in strong markets can pass $120,000.
Junior: $30,000–$60,000
Mid-level: $60,000–$100,000
Senior: $100,000–$140,000+
In the US and Western Europe, salaries often reach $60,000–$110,000. However, emerging markets may pay around $10,000–$30,000. Engineers still raise income when they build rare skills.
US / Western Europe: higher bands
Emerging regions: lower pay
Global hubs: strong packages
Final words; How Pharmuni Can Support Pharma Engineers?
Pharma engineering courses build foundations, but FDA and ICH standards keep evolving. FDA 21 CFR 210–211 and ICH Q8, Q9, Q10 define expectations for quality, risk, and control. FDA issues more than 100 of drug GMP warning letters each year, and many cite basic documentation gaps.
Pharmuni does not offer pharma engineering degrees or replace universities. Instead, our pharma engineering courses cover GMP, validation, QA, and regulatory basics that align with these guidelines. Our ISO-9001–certified system supports consistent training quality. These programs support students, recent graduates, and working professionals who want stronger compliance skills.
Therefore, Pharmuni strengthens employability with online learning and ISO-9001–backed certificates. You practise inspection-style scenarios, document clearly, and speak the language of auditors and inspectors. Many learners use these certificates to show recent, focused training alongside their degrees. Visit Pharmuni to explore pharma courses and plan your next steps.
FAQs
1️⃣ What are pharma engineering courses?
Pharma engineering courses teach how to design, run, and improve drug manufacturing processes. They mix pharmaceutical science with engineering, GMP, and quality topics.
2️⃣ Who should take pharma engineering courses?
Students in pharmacy, chemical engineering, or biotech can start building practical manufacturing skills. Working professionals in production, QA, or maintenance use these courses to upgrade and specialize.
3️⃣ Do pharma engineering courses replace a university degree?
No, they complement diplomas or degrees instead of replacing them. They add focused, industry-ready skills in areas like GMP, validation, and process control.
References

Ershad Moradi, a Content Marketing Specialist at Zamann Pharma Support, brings 6 years of experience in the pharmaceutical industry. Specializing in pharmaceutical and medical technologies, Ershad is currently focused on expanding his knowledge in marketing and improving communication in the field. Outside of work, Ershad enjoys reading and attending industry related networks to stay up-to-date on the latest advancements. With a passion for continuous learning and growth, Ershad is always looking for new opportunities to enhance his skills and contribute to pharmaceutical industry. Connect with Ershad on Facebook for more information.

Visual Inspection in Pharma (2026): What It Is, Scope, PDF Guides and SOP Essentials
Visual Inspection checks parenteral units for particles, damage, and labeling errors before release. EU GMP Annex 1 expects inspection of parenteral containers (8.30) and warns visual inspection cannot replace integrity testing, including 100% testing for fusion-sealed volumes ≤100 mL (8.22).

GxP in pharma in 2026: How Inspectors Evaluate System Control
This article explains how GxP quality systems are assessed during inspections, why gaps in governance and execution lead to repeat audit findings, and how regulated pharma operations strengthen inspection readiness through risk management, data integrity, and lifecycle compliance controls.

Quality Oversight In 2026: Roles in pharma industry, GMP Expectations, And Risk-Based Control
Quality Oversight keeps GMP decisions consistent, traceable, and risk-based. It connects deviation handling, CAPA, change control, and trend reviews. It also proves management engagement through documented actions. Strong oversight reduces repeat failures and supports confident batch disposition.


