Cover Letter still matters. With 96,472 U.S. pharmaceutical jobs and 17,251 entry-level roles on Indeed, you must stand out. Use pharma cover letter examples to tailor achievements, mirror keywords, and show motivation. In 2025, hiring leans toward skills-first, transparent processes, so personalization wins.
A strong pharma cover letter bridges résumé gaps, adds context, and proves fit beyond bullet points. It aligns your results to each company’s needs, shows domain fluency, and signals professionalism. Quantify outcomes, highlight compliance or patient-safety impact, and close with a request to interview. When recruiters stack resumes, your focused narrative gets remembered.
Table of Contents
Structure of a Successful Pharma Cover Letter
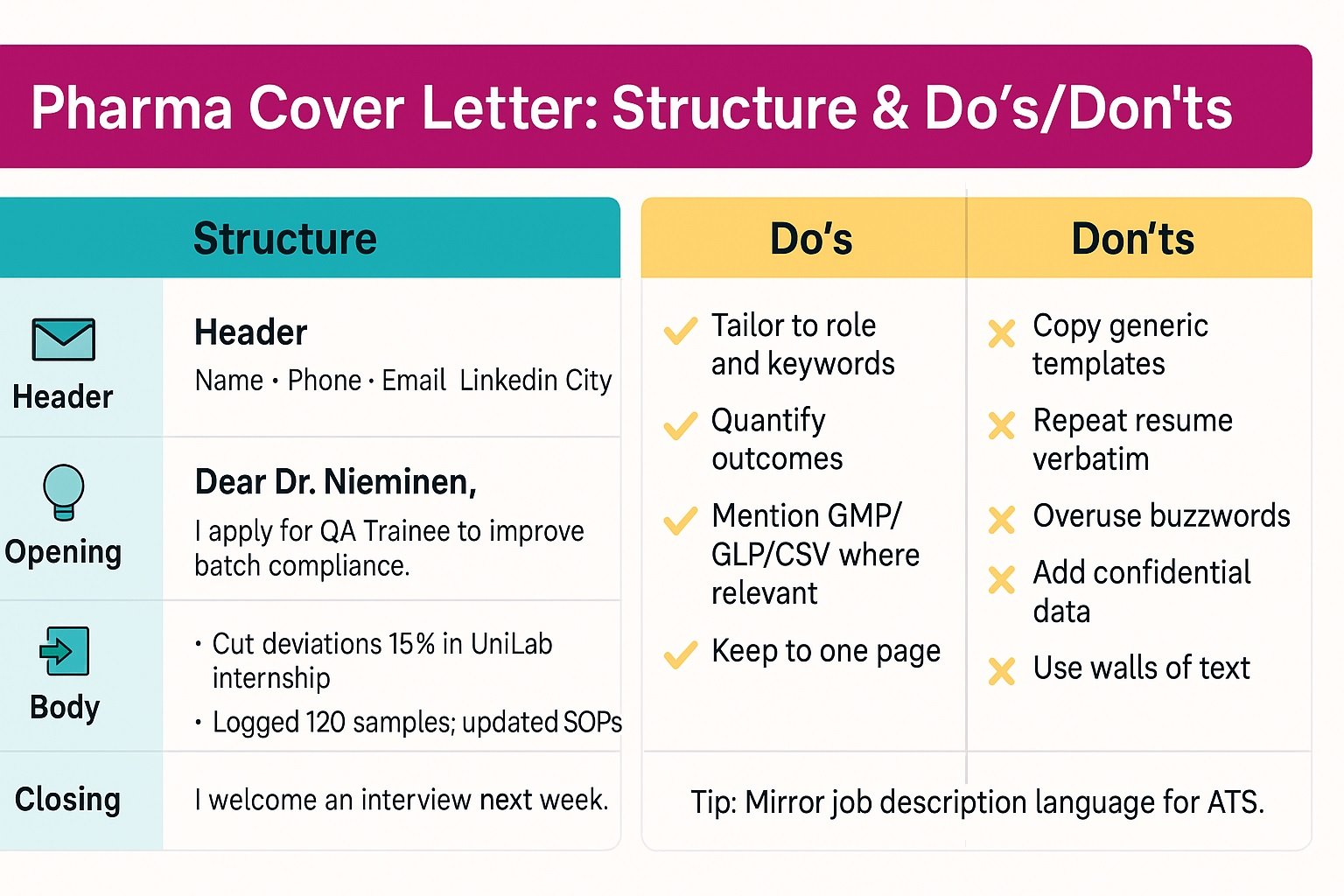
A Pharma Cover Letter needs a clear, predictable structure for busy recruiters. Use this sample cover letter for pharma freshers as your guide. First, build a concise header with name, phone, email, and LinkedIn. Then, greet the hiring manager by name when possible. Next, write an opening that states the role and your core value. Tie your background to patient safety, compliance, and product quality.
Use the body to prove fit with targeted, quantified achievements. Show domain fluency, like GMP, validation, or pharmacovigilance. Additionally, explain how your skills solve the team’s current problems. Moreover, align examples with the job description keywords. Close with a confident ask and a flexible interview time. Attach your resume and keep tone professional, warm, and direct.
Header: Name, phone, email, LinkedIn, and city
Greeting: Dear Dr. … , Hiring Manager
Opening: I apply for QA Specialist to strengthen batch compliance
Body: I reduced deviations 32% and supported three validation projects
Closing: I welcome an interview next week to discuss your goals
Pharma Cover Letter Examples (Freshers & Experienced)
Freshers show potential; experienced candidates show impact. Clarify the role and needs. Use “How to write a cover letter for pharmaceutical job” as checklist. Moreover, link achievements to patient safety and compliance.
Freshers highlight internships and certifications. Example: 12-week GMP internship, 120 samples logged, two SOPs. Experienced quantify results and audits. Example: cut deviations 32%, zero majors, three validations. Therefore, end with interview ask.
Freshers:
Internships and mini-projects show applied industry knowledge
Coursework and certifications prove real readiness
Experienced:
Metrics, audits, and submissions demonstrate measurable impact
Team leadership and mentoring highlight broad influence
A Pharma Cover Letter Example for Freshers
A Pharma Cover Letter Example for Freshers shows clarity and intent. Open with a focused job application and target role. State degree, year, and one relevant achievement. Example: QA Trainee at Orion; interned at UniLab; supported HPLC logs. Moreover, link learning to patient safety and GMP discipline.
Prove fit with a brief project and concrete numbers. Mirror keywords from the description to pass screening. Therefore, close confidently and request an interview next week. Keep tone professional, warm, and concise.
Header: Name, phone, email, LinkedIn, city
Greeting: Dear Dr. Nieminen, Hiring Manager, Orion Pharma
Opening: My job application targets QA Trainee at Orion Pharma
Education: BSc Pharmacy, 2025; coursework: GMP, QC, HPLC
Internship: UniLab intern; logged 120 samples; assisted two OOS investigations
Project: Drafted two SOPs; improved log accuracy by 15%
Skills: Excel, LIMS basics, documentation discipline, teamwork, time management
Closing: I welcome an interview next week; available Tuesday and Thursday
A Pharma Cover Letter Example for Experienced
Experienced pharma candidates must show impact, speed, reliability. Lead with strongest results. Show quality and timeline gains. Name GMP, QMS, PV, or CSV. Align achievements to the role. Keep one page. Offer flexible scheduling and clear availability.
Example: Senior QA Specialist, eight years sterile manufacturing. Cut deviations 38% in two quarters. Led three validations. Closed two audits with zero majors. Implemented CAPA tracking; release times improved 22% YoY. Targeting Site Quality Lead; interview-ready.
1. Header: Include contacts, LinkedIn, city, and best interview times
2. Opening: Name the role and your strongest quality win
3. Evidence: Present two metrics, one audit result, and one project
4. Tools: List LIMS, TrackWise, SAP, HPLC, and emerging AI aids
5. Fit: Mirror job keywords and address one clear pain point
6. Closing: Offer three time slots and request a quick meeting
Try Our Free Pharma Cover Letter Builder
Meet Pharmuni’s free Pharma Cover Letter Builder for pharma careers. Create a tailored letter in minutes, not hours. Enter your role, skills, and achievements to start. The tool guides structure, tone, and keywords for recruiters. It aligns content with GMP, QA, PV, or R&D needs. Moreover, it suggests action verbs and measurable impact statements. You keep full control and edit every field instantly.
Open Cover Letter Builder→ Create New and choose a pharma template
Fill fields: Cover Letter Name, Name, Family Name, Email, Phone, Country, Role, Body
Add job details and quantified wins; include GMP/QA/PV/R&D keywords where relevant
Preview, mirror the job description, then export your cover letter as PDF
Final Words
Customize each letter for every pharma job. Mirror job language and core competencies. Quantify outcomes that prove quality, safety, and speed. Moreover, align examples with 2025 skills-first hiring. LinkedIn reports skills-based hiring expands talent pools 6.1x globally.
Finish with clear next steps and a confident ask. Review our resume tips and interview preparation articles. Build faster using Pharmuni’s tool. Visit Pharmuni resume builder. Therefore, apply structure, metrics, and keywords to secure interviews. Proofread everything before sending.
FAQs
1️⃣ What should a Pharma Cover Letter include?
Include header, greeting, opening, body, and closing. Also add metrics, GMP terms, and role keywords.
2️⃣ How do pharma cover letter examples differ for freshers and experienced candidates?
Freshers highlight coursework, internships, and quick learning. Experienced candidates present audited results, leadership, and cross-functional wins.
3️⃣ How do I customize for each job and pass ATS quickly?
Mirror job description keywords in natural sentences to pass ATS. Alternatively, use Pharmuni’s builder to generate tailored drafts instantly.
Refrences

Ershad Moradi, a Content Marketing Specialist at Zamann Pharma Support, brings 6 years of experience in the pharmaceutical industry. Specializing in pharmaceutical and medical technologies, Ershad is currently focused on expanding his knowledge in marketing and improving communication in the field. Outside of work, Ershad enjoys reading and attending industry related networks to stay up-to-date on the latest advancements. With a passion for continuous learning and growth, Ershad is always looking for new opportunities to enhance his skills and contribute to pharmaceutical industry. Connect with Ershad on Facebook for more information.

Revalidation In Pharma (2026 guide): Meaning, Triggers, Frequency, And Requalification Differences
Revalidation protects product quality and business continuity by linking GMP decisions to risk, evidence, and context. This article helps teams decide when to act, what to document, and how to classify issues consistently before audits and regulatory inspections.

GMP Regulations in 2026: How Inspectors Assess Compliance and Control Systems
This article explains how pharmaceutical regulatory requirements shape inspection decisions, why GMP compliance gaps persist across manufacturing sites, and which operational controls, documentation practices, and risk-based measures support inspection readiness under global GMP standards.

WHO GMP in 2026: Inspection Readiness and Compliance Expectations
This article explains how global pharmaceutical GMP standards are applied during inspections, why operational gaps persist despite formal compliance, and how quality systems, contamination control, and risk-based execution shape regulatory inspection readiness across manufacturing operations.


