Pharma computer system validation (CSV) is essential for compliance in highly regulated industries. It ensures computerized systems perform reliably, securely, and consistently. Regulators such as the FDA and EMA require validation to protect patient safety and maintain data integrity. Yet, many professionals find CSV overwhelming because it involves technical steps, strict documentation, and evolving regulations.
This article breaks down CSV into clear sections. You’ll learn its purpose, regulatory expectations, best practices, and how it connects to daily work. With these insights, you’ll feel confident tackling validation challenges.

What is the purpose of pharma computer system validation?
CSV verifies that every system used in pharmaceutical manufacturing performs consistently and meets regulatory standards. The U.S. FDA 21 CFR Part 11 and EU GMP Annex 11 require documented evidence.
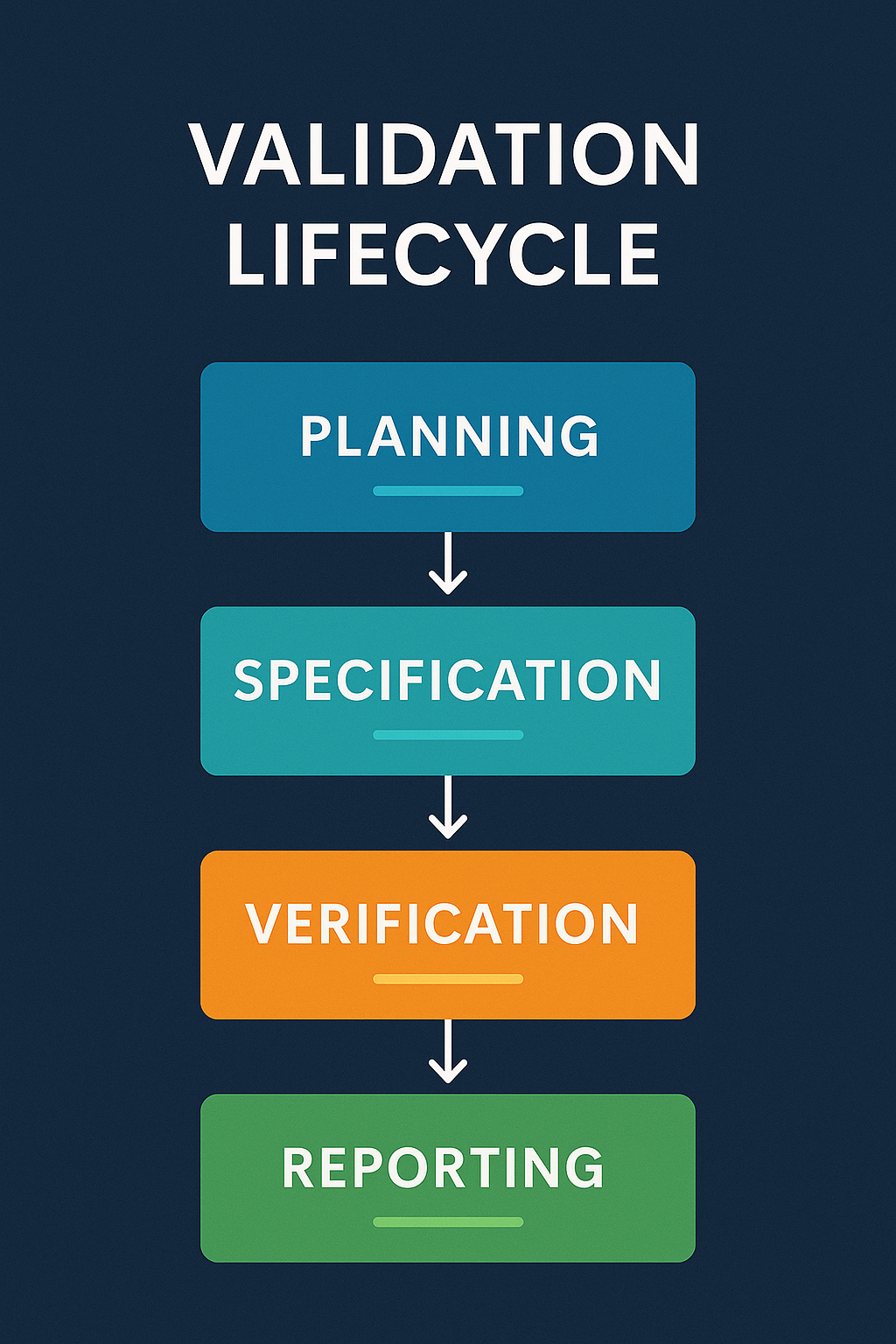
Pharma computer system validation spans the full lifecycle. It starts with user requirements, continues through design, testing, qualification (IQ/OQ/PQ), and extends to retirement.
Every stage protects data integrity and ensures decisions are made on trustworthy information.
Industry coaching helps simplify this process. For example, GMP compliance coaching teaches teams how to prepare validation protocols. A pharma career coach guides job seekers to highlight CSV experience on resumes. By combining coaching with practice, professionals gain both technical skill and regulatory confidence.
Sign up for Introduction to Computer Systems Validation Course
Why should professionals understand pharma computer system validation?
Protects patient safety
Ensures reliable systems for drug development and manufacturing.
Meets global regulations
Aligns with FDA, EMA, and ICH guidelines.
Supports audits
Creates evidence that systems are compliant and trustworthy.
Reduces risks
Prevents errors, data loss, or regulatory penalties.
Drives efficiency
Streamlines processes by standardizing validation steps.
Pharma Computer System Validation in Practice
Who benefits from mastering CSV?
Understanding CSV is vital for a wide range of professionals. Quality assurance teams rely on validation to prove compliance.
IT specialists ensure system security through validation controls. Operations staff depend on validated systems to maintain production integrity. Regulatory affairs teams need validation evidence during submissions.
Even professionals outside traditional QA roles benefit. Data analysts require validated systems to ensure accurate reporting.
Project managers depend on validation milestones to track compliance progress.
Executives use CSV results to build trust with regulators and partners.
In short, CSV knowledge makes individuals more versatile and valuable.
In a competitive pharma job market, validation skills provide a career advantage. Organizations also benefit by reducing risk, avoiding costly delays, and ensuring smooth audits.
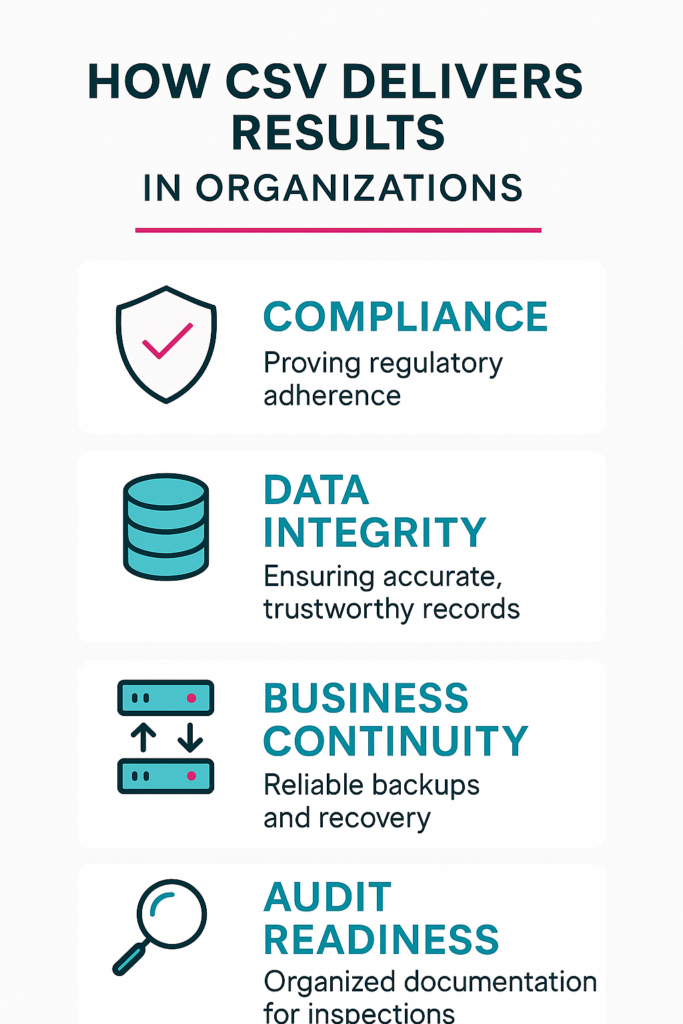
What results does CSV deliver in organizations?
Pharma computer system validation produces measurable and lasting benefits across organizations.
It strengthens regulatory compliance by delivering clear, documented evidence that every system performs as required. This compliance foundation ensures smooth submissions, faster approvals, and reduced risks of regulatory penalties.
Validation also safeguards data integrity, guaranteeing that records remain accurate, consistent, and unaltered throughout the system lifecycle.
Reliable data builds trust with regulators, healthcare professionals, and patients.
Another major benefit is business continuity. By validating backup procedures, disaster recovery plans, and access controls, companies minimize downtime and maintain productivity during unexpected events. CSV also enhances operational efficiency by streamlining processes, reducing manual errors, and standardizing workflows across departments.
Equally important, CSV builds audit readiness. During inspections, validated systems provide organized, traceable proof that processes comply with strict guidelines. This reduces stress for staff and instills confidence in regulators.
How can computerized maintenance management improve compliance and safety?
Documentation skills
Strong documentation creates a clear link between requirements, testing, and results. Well-written URS, protocols, and reports make audits faster and easier.
Testing expertise
Executing IQ, OQ, and PQ ensures systems perform as expected under real conditions. Precise testing reduces risks and proves compliance.
Risk assessment
Applying ICH Q9 methods helps identify and prioritize potential failures. Risk-based validation focuses resources where they matter most.
Audit readiness
Being prepared for inspections builds trust with regulators. Confidence during audits comes from thorough records and consistent practices.
Change management
Controlled updates prevent disruptions in validated systems. Properly documented changes maintain compliance and reduce re-validation needs.
Pharma Computer System Validation and Compliance
How does CSV impact digital systems?
Modern pharma relies heavily on digital tools. From electronic batch records to laboratory information systems, every system must be validated.
CSV ensures that these tools record, store, and transmit data securely. For example, validated audit trails prevent unauthorized changes. Validated electronic signatures ensure records are legally binding.
Validation also applies to cloud solutions. Companies must prove that SaaS platforms used in GxP processes are compliant.
How does CSV strengthen supply chain reliability?
Supply chains are complex networks where errors can be costly. Validated systems ensure reliable data flow across procurement, warehousing, and logistics.
Warehouse systems track inventory securely when validated. Procurement platforms provide trusted supplier records. Logistics tools maintain shipment traceability.
Why is pharma computer system validation a career advantage?
CSV experience makes professionals more attractive to employers. Regulatory submissions, audits, and approvals all depend on validated systems.
By mastering validation, professionals gain expertise in compliance, data integrity, and system management. This increases employability in QA, IT, and regulatory roles.
Validation also demonstrates attention to detail, risk awareness, and technical competence—qualities that employers value highly.
Where does pharma computer system validation fit in the future?
CSV continues to evolve. Emerging technologies like AI, machine learning, and advanced analytics require new validation strategies. Regulators are updating guidelines to reflect these changes, including the FDA’s Computer Software Assurance (CSA) approach.
Professionals who stay current with CSV trends will be in demand. Organizations need experts who can validate not only traditional systems but also next-generation digital platforms.
Validation remains the foundation of trust in regulated industries. As systems become more complex, its importance only grows.
FAQ: Pharma Computer System Validation
Q1. What is pharma computer system validation?
It is documented evidence that computerized systems perform reliably, securely, and in line with regulations.Q2. Why is CSV important?
It protects patient safety, ensures data integrity, and maintains regulatory compliance.Q3. Which regulations apply?
FDA 21 CFR Part 11 and EU GMP Annex 11 are the main standards.Q4. What are the main CSV steps?
Planning, requirements, design, testing, qualification (IQ/OQ/PQ), and retirement.Q5. How often should systems be reviewed?
Regulators recommend periodic review to ensure systems remain compliant.
Conclusion
Pharma computer system validation is the backbone of compliance. It protects patients, safeguards data, and ensures regulatory approval. CSV is not just a technical checklist; it is a structured process that drives trust and efficiency.
Professionals who understand validation stand out. They support audits, strengthen operations, and secure long-term career growth. Organizations that invest in strong CSV practices gain smoother inspections, reliable systems, and competitive advantage.
The future of CSV includes new approaches like Computer Software Assurance and AI validation. Staying informed ensures readiness for change.
Now is the time to act. Explore Pharmuni’s homepage, plan your future with the Career Path, and build expertise that shapes your success in pharma and life sciences.

Ershad Moradi
Ershad Moradi, a Content Marketing Specialist at Zamann Pharma Support, brings 6 years of experience in the pharmaceutical industry. Specializing in pharmaceutical and medical technologies, Ershad is currently focused on expanding his knowledge in marketing and improving communication in the field. Outside of work, Ershad enjoys reading and attending industry related networks to stay up-to-date on the latest advancements. With a passion for continuous learning and growth, Ershad is always looking for new opportunities to enhance his skills and contribute to pharmaceutical industry. Connect with Ershad on Facebook for more information.

Master GxP Validation in 2026: Meaning, Key Steps, and Validated State Control
Auditors want evidence you can trace, not opinions you can explain. GxP validation links intended use, requirements, risk, and test results into one story. When you control changes and review performance, you keep the system inspection-ready every day on time.

Master GMP Compliance in 2026: Meaning, Core Elements, and How to Implement
GMP compliance keeps medicines safe, consistent, and traceable across every batch. This guide explains core GMP elements, practical rollout steps, and common pitfalls. It also shows how to strengthen training, documentation, data integrity, and audit readiness.

History of Pharmacovigilance: From the Thalidomide Crisis (1961–2026) to GMP Oversight
Thalidomide in 1961 changed drug safety forever. Since then, pharmacovigilance has grown from crisis response to proactive risk management. Today, teams track signals, tighten reporting rules, and connect safety data to quality systems. As a result, PV now links directly to GMP oversight, audits, and data integrity.




