Media fill is the ultimate test for aseptic processes in pharmaceutical manufacturing. It simulates real production runs using growth media instead of drug product to assess contamination control. But without the right equipment, even well-planned media fills can fail.
In this guide, we reveal the essential equipment you need, common mistakes to avoid, and expert insights on regulatory compliance. If you’re involved in sterile drug manufacturing, this is your go-to resource for mastering media fills.

What Does Media Fill Really Mean in Aseptic Manufacturing?
Media fill simulates aseptic manufacturing by replacing the actual product with a microbiological growth medium like Tryptic Soy Broth (TSB). The process tests the ability of operators, equipment, and environments to maintain sterility under actual operating conditions.
Regulators such as the FDA, EMA, and WHO require media fills as a core part of aseptic validation.
According to FDA Guidance and EMA GMP Annexes for Media Fill, the procedure must replicate real manufacturing steps, simulate worst-case scenarios, and validate personnel performance.
These results must meet strict acceptance criteria. For example, the Acceptance Criteria of this process typically follow a 0.1% contamination threshold. Anything higher can trigger a full process investigation.
Sign up for Introduction to GMP Annex 1 Course
Why Is Media Fill So Important? Understanding Key Objectives
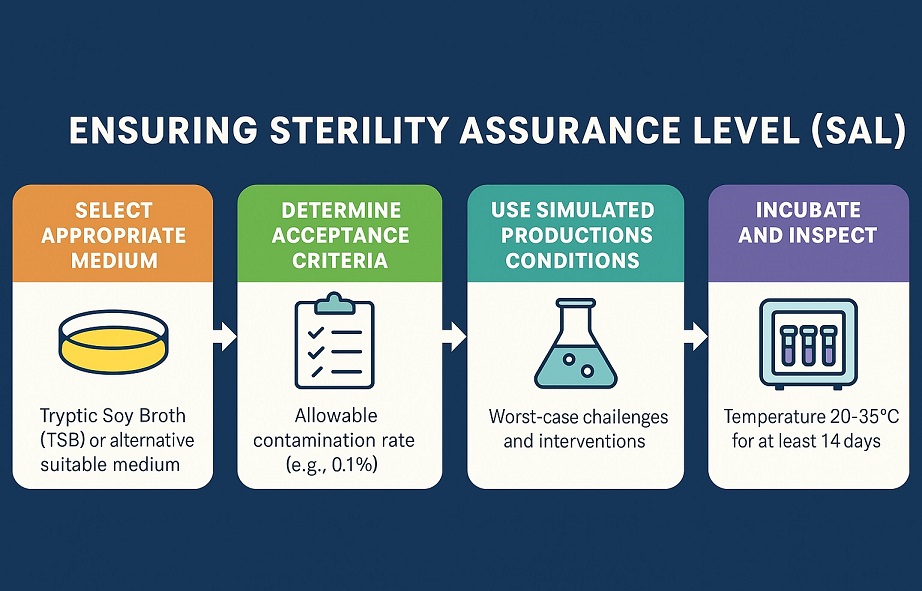
Ensuring Sterility Assurance Level (SAL)
A core objective of media fill is to ensure that aseptic processes consistently meet sterility assurance levels. ISO 13408-1 and media fill requirements define the baseline for aseptic process validation, emphasizing simulation of worst-case conditions such as manual interventions, line restarts, and extended hold times.
If contamination occurs during the fill, it means the process, environment, or personnel failed. That’s why worst-case challenges in media fill must be built into your protocol.
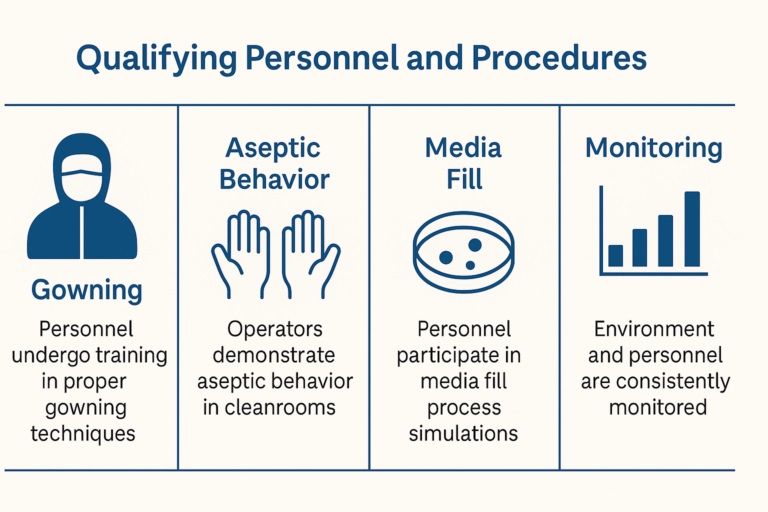
Qualifying Personnel and Procedures
Personnel qualification is essential. Operators must simulate interventions like vial capping or tubing replacement.
Their performance must be documented and reviewed. Failure to meet criteria results in retraining or process redesign.
Regulators want evidence that people, not just machines, are trained and capable.
⭐ Promote GMP Mastery: Discover our Introduction to GMP Annex 1 course to learn how environmental control, airflow, and equipment qualification intersect during aseptic manufacturing.
5 Signs Your Media Fill Setup May Be Outdated
Single-temperature incubators
No dual-stage incubation.
Lack of airborne particle monitoring
Gaps in environmental data.
Untrained visual inspectors
Miss subtle signs of turbidity.
Manual documentation
No digital audit trails.
No feedback-based training updates
Doesn’t reflect training based on media fill feedback.
How Does Media Fill Connect to Annex 1 and USP ?
Annex 1 Compliance: Environmental and Procedural Control
EMA GMP Annexes emphasize environmental monitoring during aseptic fills. It mandates active air sampling, viable/non-viable particle tracking, and contamination control strategies for RABS, LAF units, or isolators.
Key requirement: Monitoring must occur during interventions, restarts, and operator movements. Missed sampling windows may invalidate the run.
USP Media Fill Requirements
USP <797> media fill requirements focus on sterile compounding, particularly in pharmacies. Personnel must pass this test annually.
Visual inspection must occur post-incubation to spot turbidity or contamination.
Be sure to simulate worst-case: batch changeovers, stress conditions, and intervention-heavy processes.
Common Media Fill Failures and How to Avoid Them
Operator Fatigue
Leads to poor aseptic technique; rotate teams.
Hold Time Oversight
Validate worst-case line speed and hold times.
Non-qualified Equipment
Skipping calibration leads to invalid data.
Improper Container Choice
Not suitable for anaerobic media considerations.
Inconsistent Incubation
Deviations in temperature and incubation profiles.
What Are the Most Overlooked Aspects of Media Fill?
AI-powered tools now help teams detect problems during aseptic processes faster than ever. These tools track operator behavior and equipment performance in real time. They highlight abnormal actions or unexpected changes immediately. As a result, you can respond quickly and prevent contamination. Although AI isn’t mandatory yet, many companies already use it for faster root cause analysis. Moreover, it helps you improve training by identifying risky patterns in operator actions. Therefore, you can reduce human error and maintain sterility assurance. In addition, these tools support better decision-making with real-time data insights.
So, if you want to modernize your aseptic process, consider adding AI-powered detection systems.\n\nBesides that, you must keep your documentation up to date and organized. Use digital tools to log every step of your media fill run. This ensures your audit trails stay complete and easy to access.
Also, track contamination events over time using trending reports. These trends help you catch repeated issues early. Always act when values approach warning or action limits. That way, you stay compliant and avoid audit failures.
Conclusion: Master the Tools, Master the Process
Media fill is not just a box-checking activity—it’s your litmus test for sterile production integrity. From personnel qualification to environmental monitoring, every detail counts. Equip your team with best-in-class tools, validated SOPs, and consistent training. Regularly revalidate, document, and improve based on feedback.
Want to learn how regulatory expectations from GMP Annex 1 impact your daily operations? Take the next step with Pharmuni’s Introduction to GMP Annex 1 course. Get trained, get certified, and gain the confidence to lead aseptic validations.

Ershad Moradi
Ershad Moradi, a Content Marketing Specialist at Zamann Pharma Support, brings 6 years of experience in the pharmaceutical industry. Specializing in pharmaceutical and medical technologies, Ershad is currently focused on expanding his knowledge in marketing and improving communication in the field. Outside of work, Ershad enjoys reading and attending industry related networks to stay up-to-date on the latest advancements. With a passion for continuous learning and growth, Ershad is always looking for new opportunities to enhance his skills and contribute to pharmaceutical industry. Connect with Ershad on Facebook for more information.

Pharmaceutical Warehouse in 2026: GMP Storage Requirements Guide
Effective drug storage compliance depends on validated storage conditions for pharmaceuticals, structured segregation control, and reliable batch traceability in warehouse operations. This guide explains how temperature excursion management and GDP compliance warehouse practices strengthen inspection readiness and regulatory defensibility.
GMP Interview in 2026: Questions and Answers with Audit Insight
More than 60% of regulatory observations in pharmaceutical manufacturing relate to documentation control, investigation quality, and procedural compliance gaps. GMP Interview preparation in regulated environments determines whether a candidate can operate safely under inspection pressure.

Harmonization in pharmacovigilance in 2026
Inspection trends show that inconsistent global safety practices remain a leading source of pharmacovigilance findings. This article explains how regulatory harmonization, aligned safety reporting, and coordinated oversight shape inspection outcomes and support compliant international pharmacovigilance operations.

