Feeling overwhelmed by Computer System Validation (CSV)? You’re not alone. CSV compliance can feel like a maze of documents, protocols, and ever-changing regulations. In regulated industries like pharmaceuticals, failing to validate systems properly can lead to serious compliance issues. That’s why understanding the fundamentals of CSV compliance isn’t just important — it’s essential.
In this guide, we’ll break down what you need to know to take control of your CSV journey. You’ll also find real answers to your CSV questions, actionable steps, and an easy path to expertise with Pharmuni’s dedicated course.
What Does CSV Compliance Mean and Why It Matters
CSV compliance ensures that computerized systems used in pharmaceutical manufacturing are validated according to strict regulatory standards. These standards safeguard product quality, data integrity, and patient safety.
CSV compliance stems from critical regulations like 21 CFR Part 11, EU Annex 11, and GAMP 5 Guidelines. These frameworks ensure that electronic records are trustworthy and traceable. They also require proper audit trails, validation master plans, and a robust risk-based approach.
The main objective of CSV compliance is to prove — with evidence — that systems perform reliably. It involves documenting every step, from software selection to decommissioning. It’s not just an IT task. It’s a company-wide responsibility that affects QA, regulatory affairs, and manufacturing teams.
✨ Boost your confidence in CSV with our course: Introduction to Computer Systems Validation (CSV)
Sign up for Introduction to Computer Systems Validation Course
Core Components You Must Know for CSV Compliance
One of the most common pitfalls in CSV documentation is incomplete or inaccurate records. Many companies fail to capture all necessary details, leading to gaps in documentation that can raise red flags during an audit.
How to Avoid This Pitfall:
User Requirements Specification (URS)
Define what the system must do.
Functional Requirements Specification (FRS)
Describe how the system will perform each function.
4Q DQ , IQ , OQ and PQ)
Design Qualification (DQ) – Confirm that the system’s design meets requirements.
Installation Qualification (IQ) – Verify the system was installed correctly.
Operational Qualification (OQ) – Test the system’s core functionalities.
Performance Qualification (PQ) – Ensure consistent performance in real-world conditions.
Traceability Matrix
Link requirements to test cases and results.
Test Protocol Development
Write clear, structured tests for validation.
Where Does CSV Compliance Come From? A Quick Look Back
From Manual to Digital: The Birth of CSV
In the past, pharmaceutical companies used paper records to manage processes and document activities. However, as technology advanced, more systems became digital.
This shift made operations faster but also introduced new risks. Therefore, regulators started asking an important question — can we trust electronic data? As a result, the industry needed a new way to validate digital systems.
CSV, or Computer System Validation, emerged to meet this need. It helped companies show that their digital systems worked properly and recorded accurate data.
Today, CSV plays a key role in pharmaceutical compliance. It ensures digital systems are reliable, secure, and well-documented.
As more processes move online, regulators expect companies to follow strict validation steps. These steps protect data integrity and patient safety. CSV makes sure systems store information just as reliably as paper once did.
Without CSV, companies risk using systems that could produce incorrect or incomplete data. So, from paper to digital, CSV has helped the industry evolve while staying compliant and trustworthy.

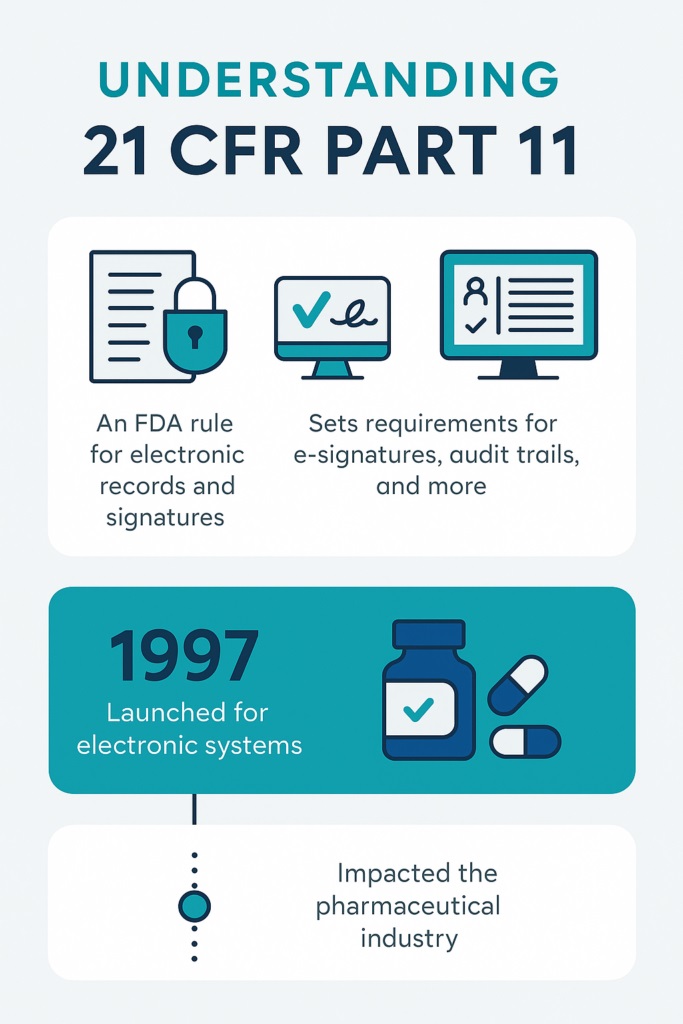
The Rise of Regulation: 21 CFR Part 11 and Beyond
In 1997, the FDA introduced 21 CFR Part 11 to control electronic records and signatures. Soon after, Europe launched EU Annex 11 with similar goals.
These regulations helped create global standards for electronic systems. As a result, companies needed to validate systems that stored critical data. CSV quickly became essential for proving system reliability and security. Today, CSV helps companies meet these strict regulatory expectations.
It connects digital innovation with compliance, creating a safe environment for patient and product data.
CSV also supports key principles known as ALCOA+. These include Attributable, Legible, Contemporaneous, Original, and Accurate data. Additionally, data must be Complete, Consistent, Enduring, and Available.
Therefore, regulators require companies to follow structured validation steps. These include audit trail reviews, risk-based methods, and strong Validation Master Plans (VMPs). Without these, data integrity may fail under inspection.
So, CSV provides a clear framework to protect data and pass audits. When followed correctly, it reduces compliance risks and builds trust in digital systems.
What Are the 7 Main Categories in CSV Compliance?
CSV compliance covers more than just software testing. It includes a complete validation lifecycle that protects your data, systems, and processes. You must understand the core categories of CSV to manage it effectively. Each category helps ensure your systems stay compliant with evolving regulations. When you follow all seven areas, you reduce the risk of audit failures and protect your company’s reputation.
Let’s break down these key categories and see how they work together.
Regulatory Frameworks and Guidelines
This category includes essential global regulations that shape CSV practices. These include 21 CFR Part 11, EU Annex 11, and GAMP 5 Guidelines. These frameworks help define what counts as a compliant digital system.
Emerging Technologies and Trends
The pharmaceutical industry is adopting new technologies fast. So, CSV must adapt to AI, Machine Learning, and Internet of Things (IoT) systems. These tools introduce automation but also increase validation complexity.
Validation Processes and Documentation
Validation relies on detailed, well-structured documentation. You must create documents like User Requirements Specification (URS) and Functional Requirements Specification (FRS). You also need Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ).
Quality Assurance and Risk Management
Quality and risk go hand in hand. You need a Quality Risk Management (QRM) plan to assess each system’s impact. You must also apply CAPA (Corrective and Preventive Actions) when something goes wrong.
Software and System Considerations
You must validate all software types, including COTS (Commercial Off-The-Shelf), SaaS (Software as a Service), and custom-built systems. Each system type brings different risks and validation needs.
Specific Applications and Systems
CSV applies to many critical systems across your company. These include Laboratory Information Management Systems (LIMS), Manufacturing Execution Systems (MES), and Enterprise Resource Planning (ERP).
Why Is Documentation So Critical in CSV Compliance
It Creates a Reliable Paper Trail
Validation documentation builds a strong and reliable paper trail for every system and process. It shows exactly what your team did and when they did it. Auditors check these records to confirm you followed every required step. Regulators also use them to assess compliance during inspections. Therefore, you must keep records that are clear, accurate, and easy to trace. Every document should link to a requirement, test, or decision. This structure gives your team confidence and reduces confusion. As a result, you avoid mistakes and improve audit readiness.
At the same time, documentation helps internal teams work together effectively. Each team member knows their role and task clearly. When teams change or grow, the records guide new staff quickly. You also spot gaps early by reviewing past validation steps. That way, you improve future projects and avoid repeated errors. So, well-managed documentation supports both compliance and performance. Without it, your team risks delays, rework, and regulatory findings. Always build your CSV process on strong documentation.

It Protects Data Integrity
Proper documentation protects your data and keeps it accurate throughout the system lifecycle. Without it, you can’t show where data comes from or how it was used.
Regulators require clear records to verify that systems handle data correctly. Therefore, you must follow guidelines like 21 CFR Part 11 to stay compliant. These rules ensure that electronic records stay complete and secure.
At the same time, good documentation helps you avoid costly errors and rework. It builds trust across teams and supports long-term success.
Moreover, data integrity depends on meeting ALCOA+ standards every day. These principles demand that data stays Attributable, Legible, Contemporaneous, Original, and Accurate.
In addition, your data must be Complete, Consistent, Enduring, and Available at all times. So, if your system lacks validation, you risk failed audits and product recalls. By keeping strong records, you show your systems work as expected.
This builds confidence with regulators and internal teams alike. Always make data integrity your top CSV goal.
What Systems Need CSV Compliance?
Pharmaceutical companies rely on several critical systems that manage essential data every day. These include Laboratory Information Management Systems (LIMS), which track and report lab samples. Next, Manufacturing Execution Systems (MES) manage batch records and oversee production activities. You also use Enterprise Resource Planning (ERP) systems to control inventory and financial data. Therefore, you must validate each system to ensure accuracy and reliability. Without validation, you risk data errors and compliance failures. Each system plays a key role in daily operations and product quality.
In addition, document and trial data must stay secure and organized. Electronic Document Management Systems (DMS) handle version control, user access, and long-term archiving. Also, Clinical Trial Management Systems (CTMS) protect patient information and maintain protocol compliance. Meanwhile, Electronic Batch Records (EBR) support good manufacturing practices and ensure reliable data. You must validate these systems to meet GMP and regulatory standards. Otherwise, you increase the risk of non-compliance and product issues. Always treat system validation as a top compliance priority.
What Mistakes Should You Avoid in CSV Compliance?
Even experienced teams make costly errors during Computer System Validation. To avoid setbacks, you must understand the most common mistakes and take steps to prevent them.
Skipping Risk-Based Assessments
You must always assess system risks before planning validation activities. High-impact systems need stronger controls and deeper documentation.
Inadequate Documentation
You must record every requirement, test, and decision in detail. Missing documents can lead to failed audits.
Lack of Training
All team members must understand their CSV responsibilities. Proper training ensures consistency and reduces validation errors.
Neglecting Change Control
Every change, even small ones, must go through a formal review. Without change control, you risk losing data integrity.
Assuming IT Owns CSV
CSV is not just an IT task — all departments must participate. Quality, regulatory, and operations teams all share responsibility.
Final Thoughts: Take Control of CSV Compliance Now
Computer System Validation is no longer optional — it’s a critical regulatory requirement. From 21 CFR Part 11 to GAMP 5, regulators expect full control over your electronic systems. But don’t worry. With the right guidance, tools, and mindset, you can master CSV compliance and avoid costly mistakes.
🔗 Ready to become a CSV expert? Take the next step with Pharmuni’s Introduction to Computer Systems Validation (CSV) course.
✨ Discover more pharma e-learning tools and resources at Pharmuni.com
References

Ershad Moradi
Ershad Moradi, a Content Marketing Specialist at Zamann Pharma Support, brings 6 years of experience in the pharmaceutical industry. Specializing in pharmaceutical and medical technologies, Ershad is currently focused on expanding his knowledge in marketing and improving communication in the field. Outside of work, Ershad enjoys reading and attending industry related networks to stay up-to-date on the latest advancements. With a passion for continuous learning and growth, Ershad is always looking for new opportunities to enhance his skills and contribute to pharmaceutical industry. Connect with Ershad on Facebook for more information.

Master GxP Validation in 2026: Meaning, Key Steps, and Validated State Control
Auditors want evidence you can trace, not opinions you can explain. GxP validation links intended use, requirements, risk, and test results into one story. When you control changes and review performance, you keep the system inspection-ready every day on time.

Master GMP Compliance in 2026: Meaning, Core Elements, and How to Implement
GMP compliance keeps medicines safe, consistent, and traceable across every batch. This guide explains core GMP elements, practical rollout steps, and common pitfalls. It also shows how to strengthen training, documentation, data integrity, and audit readiness.

History of Pharmacovigilance: From the Thalidomide Crisis (1961–2026) to GMP Oversight
Thalidomide in 1961 changed drug safety forever. Since then, pharmacovigilance has grown from crisis response to proactive risk management. Today, teams track signals, tighten reporting rules, and connect safety data to quality systems. As a result, PV now links directly to GMP oversight, audits, and data integrity.




