During modern GMP inspections, regulators increasingly shift their attention from paper-based documentation to computerized systems that generate and manage critical data. In many inspections, companies must present 12–20 controlled documents for a single system to demonstrate validation status, access control, and data integrity. This inspection reality explains why EU Annex 11 remains central to GMP compliance for computerized systems.
This annex defines how organizations must design, validate, operate, and maintain computerized systems that support GMP activities. As digital platforms now control quality records, manufacturing execution, and laboratory data, regulatory expectations have expanded accordingly. Therefore, understanding system-related compliance has become essential for inspection readiness and regulatory defensibility.
Within this context, Good Manufacturing Practices (GMP) provide the overarching framework, while Annex 11 addresses the specific risks introduced by computerized systems.
Table of Contents
What Is EU Annex 11 and Why It Matters Under GMP
Requirements for computerized systems under GMP compliance demand validated performance, reliable data integrity, and lifecycle control, making system governance a critical factor in regulatory inspections and patient safety assurance.
Unlike guidance documents, this annex carries inspectional weight. Inspectors expect clear evidence that organizations understand system risks and apply proportionate controls. Consequently, weak system governance often leads to inspection observations, even when manufacturing processes appear compliant.
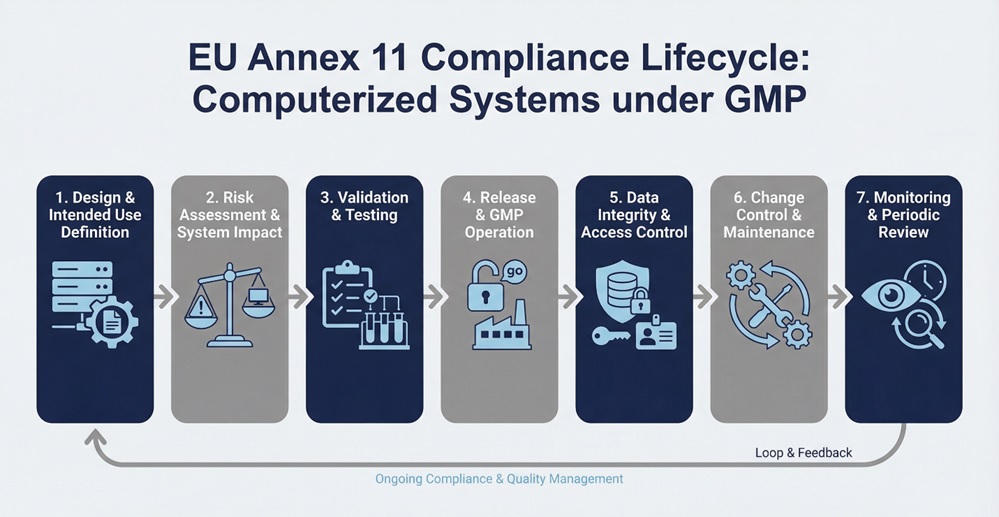
Scope of EU Annex 11 in GMP-Regulated Environments
These requirements apply whenever a computerized system supports, influences, or documents a GMP-related activity. As a result, defining system scope is not a theoretical exercise but a critical compliance decision that directly affects validation depth and regulatory oversight. If teams underestimate system relevance, they often create gaps in validation, data integrity controls, or change management that surface during inspections. Conversely, over-scoping systems can introduce unnecessary complexity, increase documentation burden, and dilute focus from higher-risk areas. Therefore, organizations must apply a risk-based approach to clearly justify which systems fall under Annex 11 and how each system supports GMP processes.
Computerized System Validation and Data Integrity Under GMP
Regulatory expectations apply to any computerized system that creates, processes, or stores GMP-relevant data. What matters is the system’s impact on quality decisions and GMP records, not its technical complexity.
In practice, Annex 11 commonly applies to:
- QMS: deviations, CAPAs, change control
- LIMS: analytical results for batch release
- MES: production steps and process data
- DMS: approved GMP documents
- GMP-relevant ERP modules: material status or release workflows
Inspectors expect clear system scoping and risk-based controls; unclear relevance often leads to inspection observations.
Regulatory Expectations for Computerized System Governance
Regulators introduced formal computerized system governance expectations to mitigate compliance risks linked to the growing reliance on digital systems in GMP-controlled operations. As pharmaceutical operations shifted from paper-based records to electronic data processing, regulators identified new vulnerabilities related to data integrity, traceability, and system reliability. Therefore, the intent of Annex 11 is not to regulate software design itself, but to ensure that computerized systems consistently support GMP principles and protect patient safety. Inspectors assess compliance by examining whether system controls effectively prevent unauthorized data changes and support reliable decision-making.
We will discuss:
- Validation expectations for computerized systems
- Data integrity and audit trail requirements
- Access control and system security
- Change control and system lifecycle management
- Supplier and third-party oversight
Validation Expectations for Computerized Systems
Regulators require documented validation to demonstrate that computerized systems operate as intended across their lifecycle. Validation must start with clear requirements and continue through testing, release, and retirement. Moreover, validation depth must reflect system risk and GMP impact.
Data Integrity and Audit Trail Requirements
Within GMP-controlled computerized systems, data integrity represents a core regulatory expectation. Computerized systems must ensure that electronic records remain complete, accurate, and attributable throughout their lifecycle. For this reason, Annex 11 requires secure audit trails that automatically record the creation, modification, and deletion of GMP-relevant data. Inspectors do not only verify that audit trails exist; they also assess whether organizations actively review them as part of routine oversight.
When audit trails remain enabled but unchecked, regulators often question the effectiveness of system control and data governance. Therefore, companies must treat audit trail review as an integral GMP activity rather than a passive system function.
Access Control and System Security
Organizations must define and control user access to prevent unauthorized activities. This includes role-based access, segregation of duties, and secure authentication. Without these controls, inspectors may question data credibility and system governance.
Change Control and System Lifecycle Management
System changes introduce compliance risk if not properly assessed. Therefore, regulated organizations must apply documented impact assessments, approvals, and testing before changes go live. approach ensures ongoing control throughout the system lifecycle.
Supplier and Third-Party Oversight
Many computerized systems rely on external vendors or cloud providers. As a result, companies must define responsibilities, assess suppliers, and maintain oversight. Regulators expect clear agreements that address data ownership, access, and availability.
Inspection Expectations for Computerized Systems in GMP Environments
During GMP inspections, authorities assess system compliance in a structured and risk-based manner. Typically, inspectors review:
- Validation documentation and lifecycle evidence
- Data integrity controls and audit trail functionality
- User access management and security settings
- Change control records and configuration history
- Backup, recovery, and business continuity arrangements
- Supplier agreements and oversight documentation
Because inspectors follow system data from creation to reporting, inconsistencies often trigger deeper review.
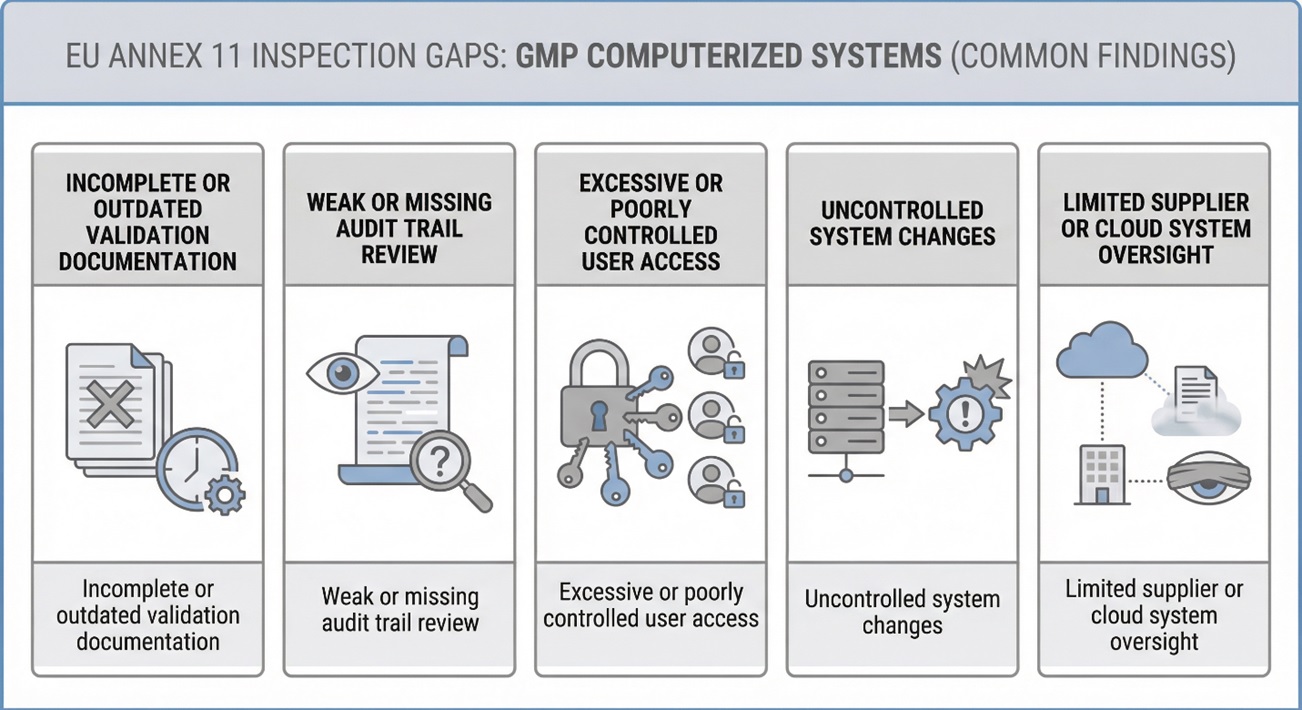
Common Compliance Gaps in Computerized GMP Systems
Inspection experience shows recurring deficiencies, including:
- Validation documentation that no longer reflects current system configuration
- Audit trails enabled but not regularly reviewed
- Excessive user privileges without justification
- System changes implemented without documented impact assessment
- Limited oversight of externally hosted or vendor-managed systems
Although these gaps vary across organizations, they usually result from unclear system ownership and weak lifecycle management.
Conclusion
In a single inspection, regulators may request 15 or more documents for one computerized system, ranging from validation reports to access logs. This level of scrutiny reflects how EU Annex 11 positions computerized system compliance as a continuous GMP responsibility rather than a one-time project. Therefore, maintaining structured system governance, clear documentation, and ongoing oversight remains essential for inspection readiness and long-term regulatory confidence.
FAQs
It requires systems that support batch processing and quality decisions to remain validated, secure, and under GMP control.
Audit trails allow inspectors to verify that critical quality data generated during testing and production remains complete, traceable, and protected against unauthorized changes.
Yes. When cloud platforms support GMP-relevant activities such as quality management, laboratory data handling, or document control, the same validation and oversight expectations apply.
References

Mahtab Shardi
Mahtab is a pharmaceutical professional with a Master’s degree in Physical Chemistry and over five years of experience in laboratory and QC roles. Mahtab contributes reliable, well-structured pharmaceutical content to Pharmuni, helping turn complex scientific topics into clear, practical insights for industry professionals and students.

Master GxP Validation in 2026: Meaning, Key Steps, and Validated State Control
Auditors want evidence you can trace, not opinions you can explain. GxP validation links intended use, requirements, risk, and test results into one story. When you control changes and review performance, you keep the system inspection-ready every day on time.

Master GMP Compliance in 2026: Meaning, Core Elements, and How to Implement
GMP compliance keeps medicines safe, consistent, and traceable across every batch. This guide explains core GMP elements, practical rollout steps, and common pitfalls. It also shows how to strengthen training, documentation, data integrity, and audit readiness.

History of Pharmacovigilance: From the Thalidomide Crisis (1961–2026) to GMP Oversight
Thalidomide in 1961 changed drug safety forever. Since then, pharmacovigilance has grown from crisis response to proactive risk management. Today, teams track signals, tighten reporting rules, and connect safety data to quality systems. As a result, PV now links directly to GMP oversight, audits, and data integrity.


