Good Manufacturing Practices are crucial in the pharmaceutical industry for ensuring quality standards. I have witnessed firsthand the positive impact GMP compliance in pharma
has had on pharmaceutical manufacturing. GMP guidelines from regulatory agencies like the FDA and EMA set the industry standard.
From raw materials to equipment maintenance, GMP covers all aspects of the production process. Compliance with GMP is more than just a formality; it prioritizes quality and safety. The FDA and EMA guidelines have improved product quality and consistency in the industry.
1️⃣ Understanding the Concept of GMP compliance in pharma
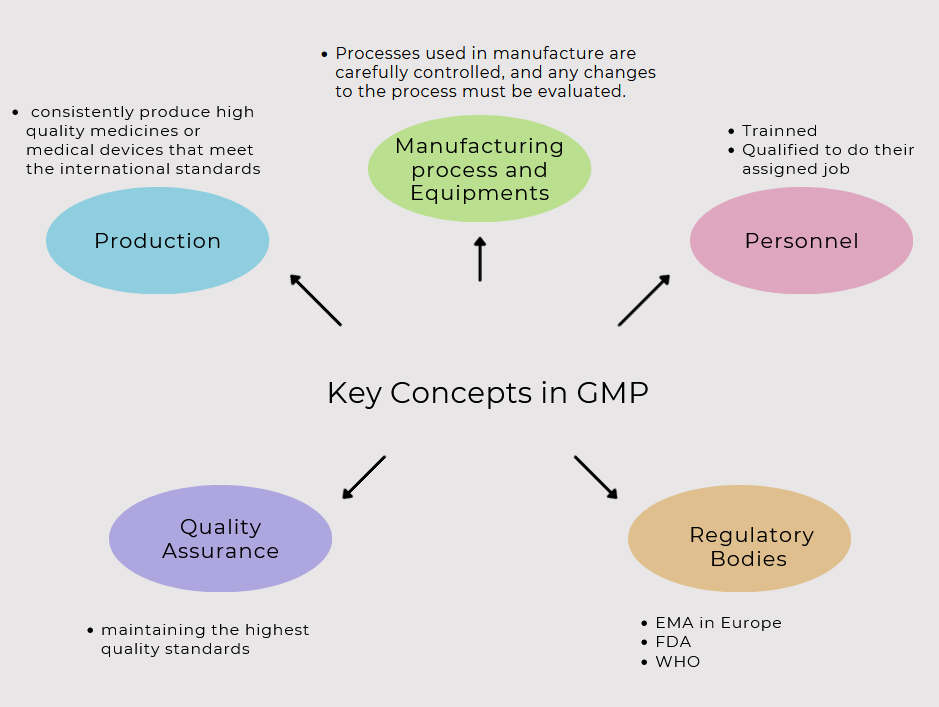
Good Manufacturing Practices, commonly , serve as the backbone of pharmaceutical industry regulations. They represent a collection of measures designed to ensure products manufactured in this industry meet the highest quality standards. Instituted by the FDA, GMP guidelines cover all aspects of production, from the quality of raw materials to the integrity of the manufacturing process and the capabilities of personnel.
Notably, GMP is not a fixed standard but a system of practices that evolve over time. This means it adapts to technological advancements, new scientific discoveries, and changing industry trends. This results in continual improvements in product safety, efficacy, and overall quality.
Within the scope of GMP compliance in pharma
, I have found that every detail matters. Even the smallest deviation from the established guidelines can lead to significant inconsistencies in the end product. This can result in a product that is ineffective or, in the worst-case scenario, harmful to the consumer.
Another critical aspect is that GMP guidelines are not only limited to the production phase but extend to the testing and quality assurance phases as well. This ensures a holistic approach towards maintaining the highest quality standards. It is a significant part of my role as a specialist to ensure these guidelines are followed meticulously at each stage.
It’s important to consider that other regulatory bodies worldwide, such as the EMA in Europe, also have their own GMP guidelines. However, they all share a common goal: to ensure the highest quality pharmaceutical products for consumers. As a specialist, keeping abreast of these guidelines, as well as any potential changes and advancements, is an essential part of my duty.
Compliance with Good Manufacturing Practice guidelines has vast implications on the pharmaceutical industry. As an industry professional, I can attest to the transformative power of these guidelines, which touch on all aspects of production, from the quality of raw materials to the training of personnel.
Adherence to this guidelines is pivotal in ensuring product quality and consistency. These guidelines obligate companies to establish robust quality management systems, validate analytical methods, and provide comprehensive documentation. The result is high-quality pharmaceutical products that meet their intended use, and importantly, earn the trust of health practitioners and patients worldwide.
GMP compliance also serves as a risk mitigation tool. The pharmaceutical manufacturing process is complex and riddled with potential risks. But by adhering to GMP guidelines, firms can prevent complications or errors, such as cross-contamination, mislabeling, and inconsistency in active ingredients. This is not only beneficial from a health perspective, but it also protects companies from the financial and reputational damage associated with product recalls and litigation.
Regulatory compliance is another crucial aspect of Good Manufactoring Practices. Regulators such as the Food and Drug Administration (FDA) in the US and the European Medicines Agency (EMA) in Europe require pharmaceutical companies to adhere to GMP guidelines. These bodies conduct regular inspections to ensure compliance, with non-compliance potentially leading to severe consequences including fines, license revocation, and criminal charges.
So, it’s clear that GMP compliance is far from just a box-ticking exercise. It is a fundamental ingredient in the recipe for a successful and responsible pharmaceutical industry. Rigorous adherence to GMP principles not only ensures the production of safe and effective medicines but also protects companies from regulatory and financial risks.
Compliance with GMP is vitally important in ensuring product quality and consistency in the pharmaceutical industry. It forms the very bedrock of the production process, requiring standards that guarantee that each product is fit for their intended use. The FDA, EMA and other regulatory bodies have clear GMP guidelines that outline the minimum requirements to ensure product safety and efficacy. From the quality of raw materials to the conditions of manufacturing and the competence of personnel, every aspect of the process is scrutinized under GMP.
Compliance with Good Manufacturing Practices (GMP) is critical in minimizing risks in the pharmaceutical industry. From my perspective as a specialist, I have seen how strict adherence to GMP guidelines can significantly reduce potential hazards that arise during the production process. These risks range from cross-contamination and incorrect labelling to the use of inadequate or excess active ingredients.
In the realm of pharmaceuticals, regulatory compliance plays a pivotal role, particularly when it comes to GMP. The Food and Drug Administration (FDA) has established GMP guidelines as a means to ensure that pharmaceutical companies consistently produce and control their products to meet quality standards. It is these guidelines that provide a foundation for the processes, procedures, and documentation that help assure the products produced have the identity, strength, composition, quality, and purity they purport to possess.
Similarly, the European Medicines Agency (EMA) has its own set of guidelines on Good Manufacturing Practice. These guidelines are harmonized to the highest possible degree with those of partner organizations in other regions, such as the FDA. Non-compliance with these guidelines might lead to a halt in production, product recall, or even legal action. As a result, understanding and adhering to these guidelines is of utmost importance for pharmaceutical companies operating in these regions. It is this adherence that ensures the integrity of the product and the safety of the patients.
3️⃣ The Future of GMP compliance in pharma
GMP compliance will continue to shape the future of the pharmaceutical industry. As companies strive to meet increasingly stringent regulatory requirements, the importance of GMP will only grow. In this rapidly evolving landscape, it’s clear that it isn’t just about avoiding regulatory fines or maintaining a good reputation. It’s about ensuring patient safety and delivering high-quality healthcare products that people can trust.
Technological advancements are playing a significant role in shaping the future of GMP compliance. Innovative technologies such as artificial intelligence (AI) and machine learning are being leveraged to streamline regulatory compliance processes, reduce human error and ultimately enhance product quality. As a specialist, being adept at using these technologies and staying abreast of the latest innovations is key to remaining competitive in this field.
However, it’s not just about embracing new technologies. The future of GMP compliance also involves fostering a culture of quality and compliance within the organization. This means not just complying with GMP regulations, but also embedding them into the organization’s ethos and everyday practices. This shift towards a more holistic approach to GMP compliance can lead to improved product quality, reduced risks, and increased customer satisfaction.
The future of GMP compliance is indeed promising. But it requires continuous efforts from us specialists, to stay informed, adapt to changes, and drive innovation. It’s a challenging journey, but one that’s integral to the evolution of the pharmaceutical industry and the health and wellbeing of patients worldwide.
Technology has always played a significant role in the pharmaceutical industry, and its importance continues to grow as we navigate the future of GMP compliance in pharma
. With the advent of groundbreaking technologies such as artificial intelligence (AI) and machine learning (ML), the pharmaceutical industry has been introduced to new possibilities for enhancing compliance with GMP regulations.
The application of AI in particular has begun to revolutionize the process of ensuring GMP compliance. AI can analyze vast amounts of data more efficiently and accurately than humans, pinpointing possible areas of non-compliance and suggesting corrective actions. This can significantly reduce the likelihood of human error, which in turn minimizes the risk of non-compliance and enhances the safety and quality of pharmaceutical products.
Machine learning, a subset of AI, is another promising technology for enhancing GMP compliance. ML algorithms can learn from past data to predict and prevent future non-compliance issues. This proactive approach can help pharmaceutical companies to stay ahead of potential issues and ensure continuous compliance with GMP regulations.
Blockchain technology is also showing potential in this arena. By providing an immutable record of all production processes, blockchain can make it easy to trace and verify compliance at every stage of the pharmaceutical production process. This can provide regulators and companies alike with the confidence that all products have been produced in accordance with GMP guidelines.
As we move forward, I believe that the adoption and implementation of advanced technologies will become standard practice in ensuring GMP compliance in the pharmaceutical industry. It’s a fascinating, transformative time, and I’m eager to see how these technologies will continue to evolve and reshape the landscape of GMP compliance.
As we look to the future of GMP compliance in the pharmaceutical industry, we cannot ignore the growing trend towards the globalization of GMP standards. I believe that the integration of global GMP standards is a crucial aspect of the pharmaceutical industry’s future. It’s not just about meeting the requirements set by different regulatory bodies, such as the FDA and the EMA. It’s about creating a unified approach to ensuring product quality, safety, and efficacy.
International harmonization of GMP standards has been a long-term goal for many regulatory bodies. For example, the International Council for Harmonisation (ICH) is working closely with various countries to align their pharma guidelines. This will streamline regulatory processes, reduce duplication of inspections, and facilitate global trade in pharmaceuticals. However, achieving this requires not only political will but also technical expertise and resources.
Implementing harmonized GMP standards across the globe will undoubtedly pose significant challenges. These range from reconciling different regulatory philosophies and practices to overcoming language and cultural barriers. Yet, despite these challenges, I believe the benefits far outweigh the costs. A unified set of GMP standards can greatly simplify compliance for multinational pharmaceutical companies, saving them time and resources.
Looking ahead, I see the potential for technology to play a crucial role in achieving this goal. Advanced technologies can aid in remote inspections, real-time quality control, and efficient data management. They can also facilitate communication and collaboration between different regulatory bodies, further promoting the harmonization of GMP standards.

CONCLUSION
GMP compliance impacts the pharmaceutical industry immensely and in various ways. It guarantees product quality, reduces risks, and meets regulatory requirements. Guidelines are enforced by FDA and EMA for public health protection.
The future of GMP compliance in pharma
will evolve with technological advancements. AI and machine learning can enhance operations and product quality. Technology will not replace humans but enhance capabilities for compliance.
Globalization is changing the dynamics of the pharmaceutical industry. Harmonizing GMP standards across regions is becoming necessary. We are moving towards universal GMP standards for competition and innovation. Future standards will safeguard public health globally.
GMP compliance ensures the safety and effectiveness of medicines. Compliance plays a crucial role in pharmaceutical manufacturing. The industry is becoming more interconnected due to globalization. Compliance guidelines are crucial in maintaining product consistency. Technology integration can streamline compliance operations and reduce errors.
Harmonization of GMP standards will foster competition and innovation. GMP standards are critical in ensuring a level playing field in the industry.
References
1-European Medicines Agency (EMA) EudraLex – Volume 4: This is THE central document for EU GMP.
2-U.S. Food and Drug Administration (FDA) Code of Federal Regulations (CFR) – Title 21, Part 210 and 211
3- ICH Q7 : Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients

Ershad Moradi
Content Marketing Specialist

Visual Inspection in Pharma (2026): What It Is, Scope, PDF Guides and SOP Essentials
Visual Inspection checks parenteral units for particles, damage, and labeling errors before release. EU GMP Annex 1 expects inspection of parenteral containers (8.30) and warns visual inspection cannot replace integrity testing, including 100% testing for fusion-sealed volumes ≤100 mL (8.22).

GxP in pharma in 2026: How Inspectors Evaluate System Control
This article explains how GxP quality systems are assessed during inspections, why gaps in governance and execution lead to repeat audit findings, and how regulated pharma operations strengthen inspection readiness through risk management, data integrity, and lifecycle compliance controls.

Quality Oversight In 2026: Roles in pharma industry, GMP Expectations, And Risk-Based Control
Quality Oversight keeps GMP decisions consistent, traceable, and risk-based. It connects deviation handling, CAPA, change control, and trend reviews. It also proves management engagement through documented actions. Strong oversight reduces repeat failures and supports confident batch disposition.