Understanding FDA vs EMA is essential for anyone working in pharma regulation. The FDA, rooted in the 1906 Pure Food and Drugs Act and formally named in 1930, oversees medicines for more than 330 million people in the US. It issues strict guidance, inspections, and warning letters that shape global pharma standards.
The EMA, founded in 1995, coordinates medicine evaluation for over 440 million EU citizens. Comparing FDA vs European Medicines Agency helps professionals navigate differing clinical, labeling, and pharmacovigilance rules. At the same time, ICH guidelines and joint inspections support global harmonization in pharma regulation.
Table of Contents
FDA vs EMA: Overview of the Two Agencies (Main Regulatory Authorities)
FDA organizes its human medicine work into key centers. CDER focuses on drugs, generics, and therapeutic biologics. CBER oversees vaccines, blood products, and advanced therapies.
-
CDER: Evaluates drug safety and effectiveness for human use.
-
CBER: Regulates vaccines, blood components, and advanced cell or gene therapies.
EMA uses an EU centralized model for many key medicines. CHMP evaluates benefits, and PRAC monitors safety signals across Europe.
-
CDER: focuses on drugs, generics, and many biologics.
-
CBER: regulates vaccines, blood, and advanced therapies.
-
CHMP: advises on benefit–risk and approvals.
-
PRAC: assesses safety data and signals.
-
EU centralized model: covers EU-wide authorization for selected products.
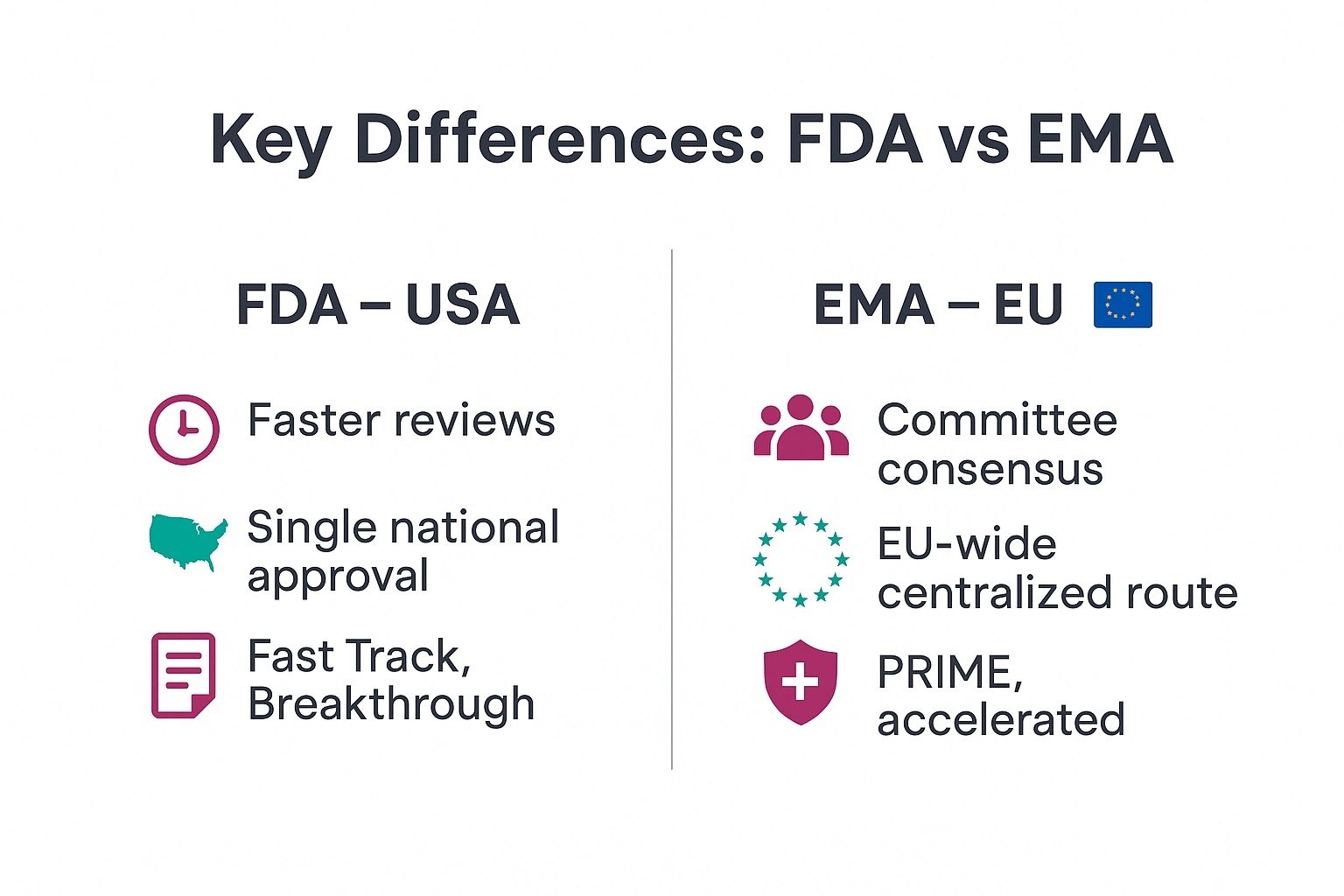
Key Differences Between FDA and EMA
Understanding FDA vs EMA helps pharma professionals plan global development and launches. . Therefore, you must know how each agency works within pharma regulation.
You compare timelines, evidence needs, and post-marketing rules before you file submissions. FDA often moves faster for breakthrough drugs, while EMA balances many national views.
Discover the real key differences between FDA and EMA.
Agency structure & committees
Review timelines
Centralized vs country-level approvals
Accelerated programs (Fast Track vs PRIME)
Labeling & post-marketing surveillance differences
Agency Structure & Committees
FDA uses centers like CDER and CBER to manage medicines. These centers report inside one agency with clear leadership lines.
However, EMA works through CHMP and PRAC as expert committees. Together they coordinate reviews for 27 EU member states.
Review Timelines
FDA sets review goals for new drugs and biologics. Standard reviews take about ten months; priority reviews aim for roughly six.
EMA uses centralised procedures with defined EU timelines for approvals. However, standard review lasts around 210 days, while accelerated assessment targets about 150.
-
FDA: Standard ≈ 10 months; priority ≈ 6 months
-
EMA: Standard ≈ 210 days; accelerated ≈ 150 days
Centralized vs Country-Level Approvals
FDA grants approvals that apply to the entire United States market. Companies then work closely with over 50 states on pricing and access.
EMA uses a centralised route that covers 27 European Union countries. However, some products still follow national pathways with extra local requirements.
Accelerated Programs (Fast Track vs PRIME)
FDA offers Fast Track to speed serious-condition drugs. It supports early talks and rolling review.
However, EMA offers PRIME to guide promising medicines earlier and coordinate EU input.
Fast Track: speeds US review with rolling submissions
PRIME: supports EU advice and faster assessment
Labeling & Post-Marketing Surveillance Differences
FDA sets detailed US labeling rules and demands clear risk communication. Companies report safety findings and adjust package inserts when new data appears.
However, EMA coordinates EU labeling through central opinions and shared safety updates. EMA committees track signals and change wording after new evaluations.
-
FDA: controls US labels and links safety reports to wording changes.
-
EMA: issues EU opinions and works with PRAC on safety-driven updates.
Which Agency Is Faster,FDA or EMA?
Comparing FDA and EMA speed helps you plan global launch timelines. Average FDA review times sit near 250 days. However, EMA reviews often approach 400 days because committees need broad agreement.
FDA uses single-agency leadership with CDER and CBER driving reviews and decisions. This structure allows quicker internal alignment for promising therapies. EMA relies on CHMP and PRAC plus member-state input. Therefore, reaching consensus can extend timelines but also strengthens shared European ownership.
For project planning, you treat these patterns as general guidance, not strict rules. You still check current pathways, because programs like Fast Track or PRIME adjust timelines.
-
FDA: typically faster median approvals and shorter priority reviews.
-
EMA: slower overall, but deeper committee debate and coordinated EU decisions.
Final Words
Pharma regulation shapes how FDA and EMA control medicines worldwide. Instead of population, we can compare their legal frameworks. FDA leans on at least four core 21 CFR parts—210–211 for GMP, 212 for PET drugs, and 314 for marketing approval.
EMA works under four cornerstone EU acts—Directive 2001/83/EC, Regulation (EC) No 726/2004, Regulation (EU) No 1235/2010, and Directive 2010/84/EU. These eight regulations create different review speeds, committee roles, and safety expectations and highlight why strong regulatory knowledge in pharma regulation matters.
Stronger regulatory knowledge protects patients and companies. It reduces findings, avoids rework, and supports launches.

Ershad Moradi
Ershad Moradi, a Content Marketing Specialist at Zamann Pharma Support, brings 6 years of experience in the pharmaceutical industry. Specializing in pharmaceutical and medical technologies, Ershad is currently focused on expanding his knowledge in marketing and improving communication in the field. Outside of work, Ershad enjoys reading and attending industry related networks to stay up-to-date on the latest advancements. With a passion for continuous learning and growth, Ershad is always looking for new opportunities to enhance his skills and contribute to pharmaceutical industry. Connect with Ershad on Facebook for more information.

GMP Environments in 2026: Inspection Readiness and Control Expectations
This article explains how inspectors evaluate GMP environments during regulatory inspections, why environmental findings often repeat, and how facility design, flow control, monitoring, and risk-based controls shape inspection readiness in real pharmaceutical manufacturing operations.

Adverse Event Reporting in 2026: Meaning, FAERS, EudraVigilance, And A Step-By-Step Guide
Adverse event reporting helps patients, healthcare professionals, and companies share safety concerns fast. Use the correct portal, submit complete case details, and send follow-up information. Clear reports support signal detection, risk review, and better pharmacovigilance decisions across systems globally today.

Master GxP Validation in 2026: Meaning, Key Steps, and Validated State Control
Auditors want evidence you can trace, not opinions you can explain. GxP validation links intended use, requirements, risk, and test results into one story. When you control changes and review performance, you keep the system inspection-ready every day on time.


