Process Validation in Pharmaceutical means using data across development and manufacture to prove a process consistently delivers quality product. FDA’s first guideline in 1987 and its lifecycle-based update in 2011 define three stages: process design, process qualification, and continued process verification, all embedded in cGMP expectations.
Pharma Validation matters because regulators link it directly to patient safety and product reliability. EMA’s EU GMP Annex 15, revised in 2015 and supported by a 2016 EMA guideline, aligns with FDA expectations by requiring risk-based, lifecycle validation documented in submissions and inspections. This framework guides modern GMP worldwide.
Table of Contents
What Is Process Validation in Pharmaceuticals?
Process validation in pharmaceuticals means you control how a process runs from start. You link science, risk management, and solid documentation. FDA and EMA define pharmaceutical validation as documented evidence that processes meet specifications. Therefore, you treat process validation as a lifecycle approach, not a single event.
Process validation stages include process design, process qualification, and continued process verification. You focus on critical quality attributes and process parameters. Moreover, you align process validation with GMP rules and inspection expectations worldwide. You may study 20–30 development batches versus 3–5 full-scale qualification batches.
Regulators expect strong pharmaceutical validation to lower risks and protect thousands of future patients. You might reduce major deviations from 4 each year to 1 after improvements. You also compare 100% critical parameter monitoring with earlier sampling checks on 10% data.
Regulatory Framework for Process Validation
Regulatory frameworks guide process validation from early design through commercial manufacturing. Authorities link validation with GMP rules to protect patients and ensure reliable quality.
Moreover, FDA guidance and EMA Annex 15 describe lifecycle expectations for process validation stages. You design, qualify, and monitor processes using science-based, risk-based principles.
Therefore, companies align internal procedures with these frameworks to meet global inspection expectations.
- FDA Guidelines (FDA 2011 Process Validation Guidance)
- EMA/EU Guidelines (EU Annex 15 Overview)
- WHO & ICH References
FDA Guidelines (FDA 2011 Process Validation Guidance)
FDA process validation guidance from 2011 explains how manufacturers design reliable processes. It links process understanding and control strategies to protect patients.
Therefore, companies follow FDA process validation stages and adjust processes when data change. Teams share findings across sites.
EMA/EU Guidelines (EU Annex 15 Overview)
EU Annex Fifteen explains how to plan process validation activities. Moreover, it links qualification steps with GMP requirements for facilities and equipment.
EMA expects firms to apply risk management when they design validation. The guideline from 2015 supports lifecycle thinking and consistent product quality.
Key points from EU Annex 15:
Define clear process validation stages and responsibilities.
Link qualification, cleaning, and transport validation.
Use documented risk assessments for critical steps.
Keep evidence ready for EMA and local inspectors.
WHO & ICH References
WHO guidelines support safe, effective medicines through modern global quality standards worldwide. Moreover, they encourage strong process validation in pharmaceutical manufacturing and distribution.
ICH documents harmonize technical expectations between major regions and regulators. They guide pharma validation strategies and lifecycle approaches for complex products.
WHO references:
WHO GMP guidelines for pharmaceutical products
WHO guidance on quality risk management and inspections
ICH references:
ICH Q8, Q9, Q10 on quality and risk management
ICH Q11 on development and manufacture of drug substances
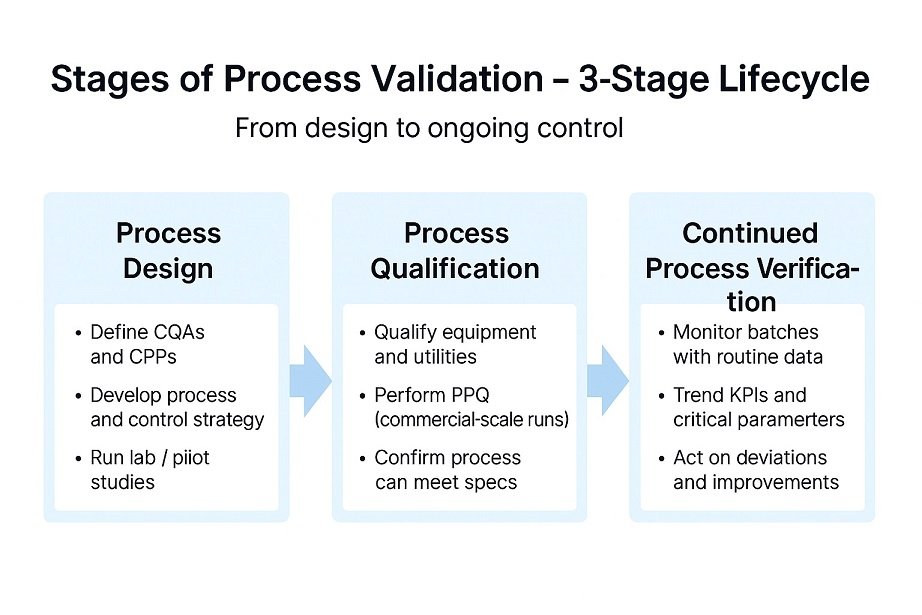
The Three Stages of Process Validation (PV Lifecycle Model)
Process validation stages cover design, qualification, and ongoing verification. You use data to keep every critical step under control.
Moreover, Stage one focuses on process design and key risks. You define parameters and link them directly to quality attributes.
Therefore, Stage two qualifies equipment and utilities for production. Stage three then verifies performance with trends and routine checks.
- Stage 1: Process Design
- Stage 2: Process Performance Qualification (PPQ)
- Stage 3: Continued Process Verification (CPV)
Stage 1: Process Design
Stage 1, Process Design, explains how the process should run. You map inputs and controls across the full PV lifecycle.
Therefore, you define critical parameters and ranges using science reliably. These choices support qualification later and enable continued process verification.
key actions
Define process steps, inputs, and equipment clearly.
Link parameters to quality attributes for later stages.
Stage 2: Process Performance Qualification (PPQ)
Stage 2, Process Performance Qualification, runs PPQ batches under routine commercial conditions. Moreover, results confirm that equipment, utilities, and controls work together within defined ranges.
Therefore, PPQ data support decisions on qualification vs validation status. Cleaning validation basics include residue limits, swab recovery, and acceptance criteria.
Qualification vs validation focus
Qualification demonstrates equipment and utilities meet predefined installation and operational criteria.
Validation confirms the end-to-end process meets consistent quality across PPQ batches.
Cleaning validation basics
Identify worst-case products, soils, and equipment surfaces for PPQ evaluations.
Establish sampling points, analytical methods, and residue limits for verified cleanliness.
Stage 3: Continued Process Verification (CPV)
Stage 3, Continued Process Verification, monitors performance data over time. Teams track batches and deviations in routine manufacturing.
Moreover, CPV identifies trends early and supports process adjustments. Strong monitoring maintains control and protects patient safety.
Digital Tools & Modern Trends in PV
Digital tools change process validation in modern pharma plants. Data historians collect continuous batch data and support faster reviews.
Moreover, advanced analytics detect small shifts before they cause deviations. Cloud platforms store validation reports and link them with equipment records.
Therefore, teams use digital twins and eBR systems to test scenarios. Mobile apps guide process validation stages and capture checks in real time.
Digital Tools & Modern Trends in PV (2026)
Digital tools transform process validation in 2026 across development and manufacturing. Data historians collect continuous parameters and support faster investigations and reviews. Moreover, advanced analytics flag subtle trends before deviations impact critical quality attributes. Therefore, teams integrate sensors, MES, and LIMS into one real-time monitoring ecosystem.
Modern systems also streamline validation documentation pharma requirements globally. Structured templates, e-signatures, and audit trails strengthen data integrity and traceability. Additionally, cloud platforms centralize protocols, reports, and CPV dashboards for global sites.
Final words
Process Validation in Pharmaceutical links science, risk, and data across the whole lifecycle. It follows FDA 2011 guidance and ICH Q8, Q9, and Q10. Moreover, it uses a validation master plan to align goals, scope, and responsibilities.
Teams write each validation protocol to define tests, batches, and acceptance criteria. They collect data from Stage 1 to Stage 3 and review trends. Therefore, they support safe, consistent products for millions of patients worldwide.
To grow skills, professionals explore pharma courses on validation and GMP. They study lifecycle case studies, modern digital tools, and global inspection expectations.
FAQs
1️⃣ What are the three stages of process validation?
Stage 1: Process Design defines how the process should run.
Stage 2 and 3: PPQ and Continued Process Verification confirm and monitor performance.
2️⃣ Why does Stage 1: Process Design matter?
It links materials, equipment, and parameters to product quality.
It creates the scientific basis for all later validation work.
3️⃣ How do Stage 2 and Stage 3 support routine manufacturing?
Stage 2 PPQ shows the process runs consistently at commercial scale.
Stage 3 CPV tracks trends, deviations, and improvements over time.
References

Ershad Moradi, a Content Marketing Specialist at Zamann Pharma Support, brings 6 years of experience in the pharmaceutical industry. Specializing in pharmaceutical and medical technologies, Ershad is currently focused on expanding his knowledge in marketing and improving communication in the field. Outside of work, Ershad enjoys reading and attending industry related networks to stay up-to-date on the latest advancements. With a passion for continuous learning and growth, Ershad is always looking for new opportunities to enhance his skills and contribute to pharmaceutical industry. Connect with Ershad on Facebook for more information.

GMP Environments in 2026: Inspection Readiness and Control Expectations
This article explains how inspectors evaluate GMP environments during regulatory inspections, why environmental findings often repeat, and how facility design, flow control, monitoring, and risk-based controls shape inspection readiness in real pharmaceutical manufacturing operations.

Adverse Event Reporting in 2026: Meaning, FAERS, EudraVigilance, And A Step-By-Step Guide
Adverse event reporting helps patients, healthcare professionals, and companies share safety concerns fast. Use the correct portal, submit complete case details, and send follow-up information. Clear reports support signal detection, risk review, and better pharmacovigilance decisions across systems globally today.

Master GxP Validation in 2026: Meaning, Key Steps, and Validated State Control
Auditors want evidence you can trace, not opinions you can explain. GxP validation links intended use, requirements, risk, and test results into one story. When you control changes and review performance, you keep the system inspection-ready every day on time.


