Internships change careers for pharma students. Many students understand theory. However, employers want proof of real skills. Internships give that proof and show how pharma workplaces actually run. Many pharma student internships also open doors to first jobs.
Today you can choose from several structured options. In this guide, we focus on six core types of internship for pharma students: industrial/plant, QA/QC, R&D/lab, pharmacovigilance and drug safety, regulatory affairs, and medical affairs. These categories support different goals: industry roles, clinical paths, research careers, regulatory tracks, or safety-focused jobs.
Therefore, you should not pick internships randomly. Instead, you match each option to your long-term plan. Many pharma students want jobs in production or quality. Others prefer research labs, drug safety, or regulatory strategy. Each internship builds different skills and CV stories.
What are Pharma Student Internships?
Pharma student internships are short, structured work experiences in pharmaceutical companies, hospitals, CROs, or research centers. You usually join for 2–6 months. Sometimes programs run over a full year.
You handle real tasks under supervision. For example, you support batch documentation, assist in stability studies, screen adverse event reports, or help prepare regulatory files. Managers give feedback and track your progress.
Moreover, these internships do three important things:
- They test your career fit before you commit.
- They build hands-on skills beyond classroom work.
- They help you grow a network inside the pharmaceutical ecosystem.
Many companies now treat pharmacy student internships as a talent pipeline. Therefore, strong performance can lead to job offers, thesis topics, or future research collaborations.
What are Pharma Student Internships?
You now see many pharma internship types in job portals and university boards. However, the names sometimes confuse students. So let’s group the main categories and keep things clear.
Most pharmaceutical internships for students fall into these six types:
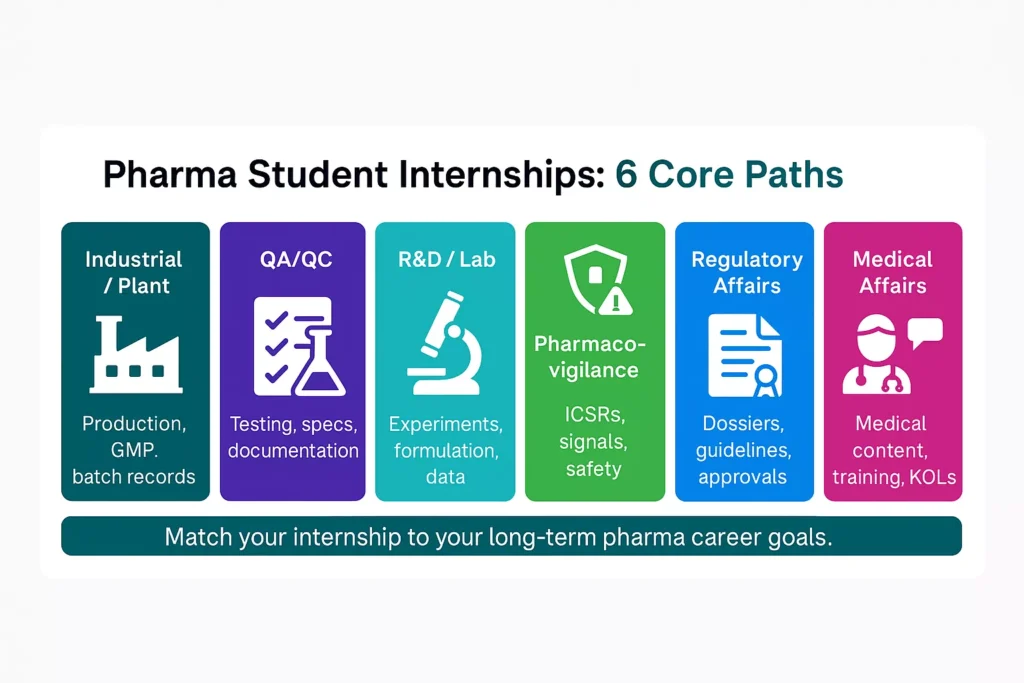
You may also find industrial pharmacy internship or hospital pharmacy internship postings. These often align with the categories above but focus more on manufacturing or clinical environments.
Industrial / Plant
Industrial or plant internships show you how real medicines leave the factory line.
- Industrial or plant pharma student internships suit you if you enjoy fast-paced factory environments.
- Observe full production lines and follow tablets or injectables from raw material to final pack.
- Support operators with batch records, line clearance checks, and basic equipment start-up routines.
- Practice time management, attention to detail, and clear communication with technicians and supervisors.
- Remember one tip: always ask about GMP rules before touching equipment or documents.
QA/QC
QA/QC internships focus on quality, details, and documentation.
- Join quality teams to handle samples, label vials, and prepare materials for testing.
- Review simple lab results and compare them with specifications under guidance from analysts.
- Develop strong documentation habits, GMP awareness, and basic problem-solving around out-of-trend results.
- Focus on one key tip: never skip signatures, dates, or units when you write in lab records.
R&D / Lab
R&D or lab internships suit curious students who enjoy experiments.
- Assist scientists with weighing, dissolving, mixing, and recording data for small development batches.
- Explore formulation ideas, stability trials, and method comparisons in a structured project plan.
- Strengthen skills in GLP, data analysis, and scientific reading for future master’s or PhD plans.
- Keep one tip in mind: write everything in your notebook immediately, not hours after the experiment.
Pharmacovigilance & Drug Safety Internships
Pharmacovigilance internships connect real patients, data, and medicine safety.
- Handle basic case entry, check completeness, and update safety databases with supervised support.
- Read narratives to link symptoms, treatments, and timelines in a logical sequence.
- Build skills in medical terminology, critical thinking, and clear English writing for safety reports.
- Apply this tip: always clarify unclear information instead of guessing in an adverse event file.
Regulatory Affairs
Regulatory affairs internships blend science with rules and clear writing.
- Collect data from QA, R&D, and PV teams for variations, renewals, or new submissions.
- Compare product information with guidelines and note gaps that need clarification.
- Improve skills in technical writing, guideline reading, and cross-functional communication.
- Use one tip consistently: keep a personal glossary for key regulatory terms and acronyms.
Medical Affairs
Medical affairs internships focus on scientific communication and education.
- Draft simple slide decks, FAQs, and email updates based on clinical study results.
- Talk with cross-functional teams to align messages for sales, marketing, and regulatory colleagues.
- Sharpen skills in storytelling, literature search, and explaining complex data in plain language.
- Follow this tip: always check every claim against the approved label before you add it to materials.
How to Choose the Right Student Internship Based on Your Pharma Career Goal
You now know the main internship types. However, you still need a clear choice. So use these five points as a quick decision checklist:
- Clarify your long-term direction: Decide if you prefer industry, research, clinical, regulatory, or safety roles. Then pick internships that move you one step closer.
- Match tasks to your strengths: If you enjoy lab work, choose R&D or QC. If you love documents and rules, pick regulatory or PV.
- Check learning goals, not just brand names: Big companies impress, but small firms often give broader tasks. Therefore, always read the task list carefully.
- Look at supervision and feedback: Strong mentors improve growth. So ask how often you receive feedback and who guides you.
- Think about the next two steps: Ask how each program helps your thesis, first job, or future specialization. For example, an industrial internship plus a QA internship can create a powerful production-quality profile.
When you compare offers, write a short table. Include type, location, skills, supervision level, and growth potential. Then choose the internship that best supports your next three to five years.
Final words
Pharma student internships give more than experience. They test your interests, build confidence, and turn theory into real skills. Even one strong internship can boost your hire chances by a huge margin. Two or three targeted roles across 2–3 years can show a clear story: where you started, what you learned, and how you grew.
You now know six core paths: industrial/plant, QA/QC, R&D, pharmacovigilance, regulatory affairs, and medical affairs. Next, connect these options to your long-term goals. Then map your skills to real roles and plan your next move. To design that journey step by step, Explore Pharma Career paths and turn each internship into a powerful career milestone.
FAQ:
Pharma internships give you short, structured work experience in the pharmaceutical field. You join real teams in industry, hospitals, or research labs. During the internship, you learn practical skills, build contacts, and test your career interests.
You usually apply 3–6 months before the start date. Many companies open internship for pharma students during fixed seasons, such as summer or the final study year. Therefore, you should check university boards and company pages early. You then plan your exams, thesis, and travel around the internship period.
Internships translate theory into real skills. You learn how teams work, how documents flow, and how decisions happen. More importantly, you gain references and contacts. Many companies later hire strong interns into entry-level roles or support their thesis projects.
Some pharmaceutical internships for students offer payment or a stipend. Others only cover meals or transport. A few remain unpaid. Therefore, you should always ask about payment, benefits, and working hours before you accept. You then compare total value, not just money.
References:

Stephanie Männicke
Digital Marketing Especialist at Zamann Pharma Support, brings 8 years of experience in Corporate and Digital Communication. Specializing in Digital Marketing and Content Creation, Stephanie is currently focused on creating strategic content for Pharmuni's networks, especially content on topics such as recruitment, onboarding and employer branding. Outside of work, Stephanie is a mum, a crocheter and a movie fan. An avid reader and in search of expanding her knowledge, Stephanie is always looking for ways to innovate communication in the digital environment and connect people in a genuine way.

Revalidation In Pharma (2026 guide): Meaning, Triggers, Frequency, And Requalification Differences
Revalidation protects product quality and business continuity by linking GMP decisions to risk, evidence, and context. This article helps teams decide when to act, what

GMP Regulations in 2026: How Inspectors Assess Compliance and Control Systems
This article explains how pharmaceutical regulatory requirements shape inspection decisions, why GMP compliance gaps persist across manufacturing sites, and which operational controls, documentation practices, and

WHO GMP in 2026: Inspection Readiness and Compliance Expectations
This article explains how global pharmaceutical GMP standards are applied during inspections, why operational gaps persist despite formal compliance, and how quality systems, contamination control,


