Pharmacovigilance salary have become a hot search topic. Fresh graduates want to know if a PV salary can support their goals. Mid-career professionals want to compare drug safety salary packages across regions and industries.
The global Pharmacovigilance market keeps growing fast. Analysts expect the PV market to reach around USD 18 billion by 2030, with strong double-digit growth from 2025 onward. Therefore, companies need more drug safety professionals than ever. Regulators push for stronger safety systems. Moreover, new therapies, biologics, and global trials create more data and more risk signals.
In this guide, you discover how salaries vary by experience, job role, location, and employer type. You also see how to use skills and learning to improve your long-term earning potential in global PV.
Factors Influencing Pharmacovigilance Salary
Many elements shape a drug safety salary. You control some of them; others depend on the market. However, you should understand all of them before you negotiate.
Geography and cost of living
Location plays a huge role.
- In high-income regions like the US and Western Europe, PV salaries look higher in absolute numbers.
- In India and parts of Asia, salary levels sit lower yet living costs also stay lower.
- In the Gulf region, base pay often combines tax advantages and housing benefits.
Therefore, always compare net income and living costs together, not only headline numbers.
Company type: Pharma, biotech, CRO, or BPO
Your employer type also impacts PV salary:
- Global pharma and large biotech often pay higher, especially for complex roles like signal detection or medical reviewer.
- CROs and specialized PV vendors offer strong learning exposure and fast promotion paths.
- BPOs and shared service centers may pay less per year but provide many entry-level roles for pharmacovigilance salary for freshers.
Role complexity and responsibility
More complex pharmacovigilance job roles usually earn more:
- Case processing and ICSRs sit at the entry level.
- Aggregate report writing (PSUR/DSUR), signal detection, and global PV strategy sit at mid to senior levels.
- PV quality, audits, and global safety lead roles carry strong responsibility; therefore, they command higher pay.
Experience level and certifications
Experience remains a key driver. A senior PV scientist or PV manager earns far more than a fresher. Certifications and advanced degrees also help:
- PharmD, MD, or strong clinical background.
- PV diplomas, GCP certificates, or regulatory affairs training.
- Specialized courses in signal detection, case processing, and regulatory compliance.
In addition, niche experience in risk management plans, labeling, or global PV systems can lift your PV salary.
Pharmacovigilance Salary by Experience Level
Now let us look at PV salary by seniority. These numbers are global ballpark ranges in gross annual salary. They vary widely by region and company, so treat them as approximations.
PV salary for freshers (0–2 years)
Freshers usually work as drug safety associates or junior PV officers. They focus on case processing, data entry, coding, and narrative writing.
Approximate global ranges in 2025:
- India: USD 5,000–10,000 per year (≈ ₹4–8 lakh)
- Europe: EUR 30,000–45,000 per year for PV associates
- US: USD 45,000–65,000 per year for PV associates
- Middle East (UAE/Saudi): USD 30,000–45,000 per year, sometimes tax-free
Therefore, pharmacovigilance salary for freshers gives a solid entry point, especially when you add shift allowances and bonuses.
Early to mid-career PV salary (3–7 years)
At this stage, you may work as a senior associate, case processing lead, or aggregate reporting specialist. You handle complex ICSRs, mentor juniors, and support audits.
Typical ranges:
- India: USD 10,000–20,000 per year (≈ ₹8–16 lakh).
- Europe: EUR 45,000–65,000 per year.
- US: USD 70,000–100,000 per year for safety and pharmacovigilance specialists.
- Middle East: USD 50,000–70,000 per year for PV specialists, plus benefits.
Senior salary (8+ years / management)
Senior professionals work as PV scientists, medical reviewers, safety leads, or PV managers. They handle signal detection, aggregate reports, benefit-risk evaluation, and global PV strategy.
Approximate ranges:
- India: USD 20,000–40,000 per year (≈ ₹16–32 lakh+), depending on role and company.
- Europe: EUR 70,000–120,000 per year for PV managers and heads.
- US: USD 110,000–170,000+ per year for senior safety leaders.
- Middle East: USD 80,000+ per year for senior roles in global pharma subsidiaries.
Therefore, experience plus leadership responsibility can double or triple your PV salary over time.
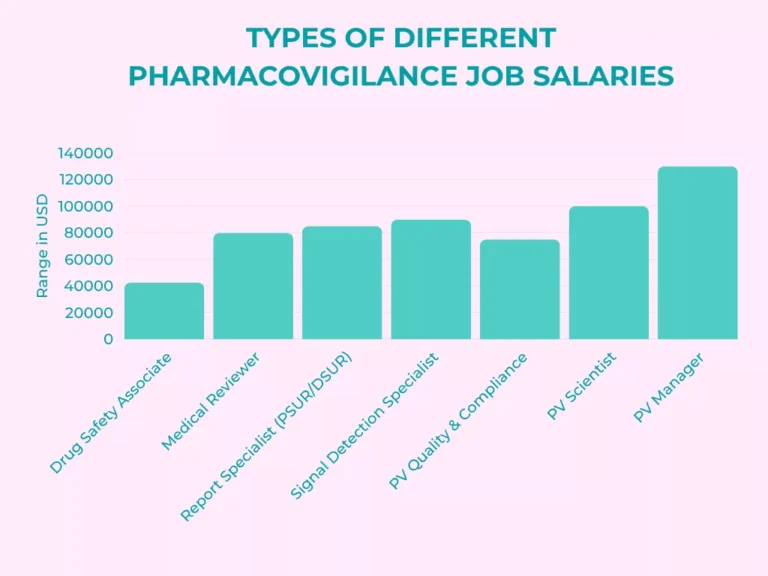
Pharmacovigilance Salary by Job Role
Here you see how pharmacovigilance job roles compare. Again, these numbers reflect global averages and convert everything roughly into annual USD for easier comparison.
Drug Safety Associate (ICSR / Case Processing)
- Case intake, MedDRA coding, narrative writing, follow-up.
- USD 25,000–60,000 globally, from India to US and EU.
Medical Reviewer
- Medical assessment of ICSRs, seriousness and causality, label impact.
- USD 50,000–110,000 depending on region and whether you hold an MD or PharmD.
Aggregate Report Specialist (PSUR / DSUR / RMP)
- PSURs, DSURs, RMPs, benefit-risk evaluation, signal interpretation.
- USD 55,000–115,000 globally.
Signal Detection Specialist
- Statistical signal detection, database review, literature, dashboards, cross-functional review.
- USD 60,000–120,000; top global pharma roles may exceed this range.
PV Quality & Compliance / PV Officer
- Audits, SOPs, CAPA, inspection readiness, vendor oversight.
- USD 50,000–100,000 globally; in the UAE and Europe, strong inspection experience can push this higher.
PV Scientist / Safety Scientist
- Advanced signal work, clinical review, risk management, labeling, cross-functional safety strategy.
- USD 70,000–130,000, especially in US and Western Europe.
PV Manager / Global PV Lead
- Team leadership, global PV systems, budget, vendor and partner oversight, regulatory interaction.
- USD 90,000–170,000+ in mature markets; senior heads in big pharma may earn more.
As you move from PV officer salary levels into scientist and manager roles, you handle more cross-functional work with clinical, regulatory, and medical affairs. Therefore, leadership and communication skills become as important as technical knowledge.
Global Comparison
Now let us compare salary trends across key regions: India, the US, Europe, and the UAE. The cost of living and tax rules shape these differences.
India: Strong entry hub for PV
India acts as a global hub for case processing and PV outsourcing. Therefore, it offers many entry-level roles and clear growth paths.
- Drug safety associate average salaries sit around ₹4–5 lakh per year in many companies, with higher levels for experienced staff.
- Senior roles and team lead in metro cities can cross ₹15–20 lakh per year.
Living costs are lower than in Western countries, so purchasing power can still look attractive. However, remote and hybrid work may create new benchmarks over time.
United States: High absolute pay
The US shows some of the highest PV salary levels. Large pharma and biotech clusters in Boston, New Jersey, and California drive demand.
- PV associates usually earn USD 45,000–65,000.
- Safety and pharmacovigilance specialists average close to USD 98,000.
- Experienced pharmacovigilance specialists often report ranges of USD 70,000–85,000 or more.
However, major US cities also bring high housing and healthcare costs, so net savings require planning.
Europe: Stable, regulated, and diverse
Europe includes many PV hubs, especially in Germany, the Netherlands, Ireland, and the UK.
- PV associates in the Netherlands start around EUR 33,000–48,000.
- In Germany, PV managers often earn EUR 70,000–120,000, while mid-level professionals sit around EUR 50,000–90,000.
Moreover, social benefits and work–life balance add hidden value, even if nominal salaries look lower than in the US.
UAE and Gulf: Tax benefits and allowances
In the UAE and wider Gulf region, drug safety salary packages often include tax advantages, housing, and relocation benefits.
- Drug safety or PV specialists may earn USD 50,000–70,000 per year, plus benefits.
- In Dubai, salary surveys show wide ranges for drug safety specialists, with many professionals earning over 12,000 AED per month.
Therefore, always evaluate base pay, tax rules, and benefits together when you compare offers between the Gulf and other regions.
Final Words
Pharmacovigilance offers a stable, long-term career with clear salary growth. You can start in case processing or as a drug safety associate, then move into aggregate reporting, signal detection, or medical review. As you gain experience, you can progress into PV scientist or PV manager roles and handle global safety strategy. Therefore, each step brings more responsibility and stronger earning power. At the same time, demand for drug safety skills keeps rising as companies launch more complex therapies and expand into new markets.
However, your pharmacovigilance salary will depend strongly on your skills and actions. You should build a solid base in ICSRs, MedDRA coding, PSURs, and regulatory compliance. Then you can add advanced skills in signal detection tools, inspection readiness, and cross-functional communication. Moreover, you can use specialized online courses, PV career paths, and certifications to close gaps and negotiate better offers in regions like India, Europe, the US, or the Gulf.
FAQ:
Pharmacovigilance salary depends on the country, employer, and experience. Freshers often start with a modest PV salary, while senior PV managers and scientists earn significantly more. Therefore, you should always compare offers with local living costs and benefits.
Pharmacovigilance salary for freshers usually starts at the drug safety associate or PV officer level. You handle basic case processing, data entry, and MedDRA coding, so you gain strong foundations. Over time, you can grow your PV salary by moving into more complex tasks and taking on extra responsibilities.
The highest pharmacovigilance salary usually appears in senior roles like PV manager, safety lead, medical reviewer, and global PV scientist. These positions involve strategic decisions, signal detection, PSUR oversight, and regulatory compliance leadership. Therefore, they reward strong experience, leadership, and cross-functional skills.
You can increase your pharmacovigilance salary by building niche skills and certifications – try Pharmuni’s Pharmacovigilance Course. Focus on areas like signal detection, PSUR and DSUR writing, medical review, and regulatory compliance. In addition, you can learn about global PV regulations and lead cross-functional projects to show higher value to employers.
References:

Stephanie Männicke
Digital Marketing Especialist at Zamann Pharma Support, brings 8 years of experience in Corporate and Digital Communication. Specializing in Digital Marketing and Content Creation, Stephanie is currently focused on creating strategic content for Pharmuni's networks, especially content on topics such as recruitment, onboarding and employer branding. Outside of work, Stephanie is a mum, a crocheter and a movie fan. An avid reader and in search of expanding her knowledge, Stephanie is always looking for ways to innovate communication in the digital environment and connect people in a genuine way.

Master GxP Validation in 2026: Meaning, Key Steps, and Validated State Control
Auditors want evidence you can trace, not opinions you can explain. GxP validation links intended use, requirements, risk, and test results into one story. When

Master GMP Compliance in 2026: Meaning, Core Elements, and How to Implement
GMP compliance keeps medicines safe, consistent, and traceable across every batch. This guide explains core GMP elements, practical rollout steps, and common pitfalls. It also

History of Pharmacovigilance: From the Thalidomide Crisis (1961–2026) to GMP Oversight
Thalidomide in 1961 changed drug safety forever. Since then, pharmacovigilance has grown from crisis response to proactive risk management. Today, teams track signals, tighten reporting


