Pharma regulatory bodies like the FDA and EMA protect patients by enforcing pharma regulation that demands proven safety and efficacy. They review data, factories, and post-market reports. WHO has 194 Member States, yet only about 26% have fully functioning regulators, so strong oversight still does not reach every population.
Globally, harmonisation keeps growing. WHO has now listed 36 regulatory authorities as WHO Listed Authorities, encouraging others to align with their standards. Mutual recognition deals, such as FDA–EU inspection agreements, help agencies rely on each other’s work and speed access to quality medicines without lowering safeguards.
Table of Contents
What Are Pharma Regulatory Bodies?
Pharma regulatory bodies are official organizations that oversee medicines from lab to patient. Pharmaceutical regulatory agencies set rules for research, manufacturing, labeling, marketing, and post-market monitoring. They check if companies follow laws and keep drug information honest and complete.
Drug regulatory authorities protect public health by reducing risks from unsafe or poor-quality products. Moreover, they review clinical trial data and inspection findings before they approve new medicines. Therefore, they help people trust treatments and support fair access to essential therapies.
Core activities of these bodies include:
Review medicine safety, quality, and efficacy data
Inspect factories, labs, and distribution sites regularly
Enforce regulations and respond quickly to safety concerns
Educate healthcare professionals and the public about safe medicine use
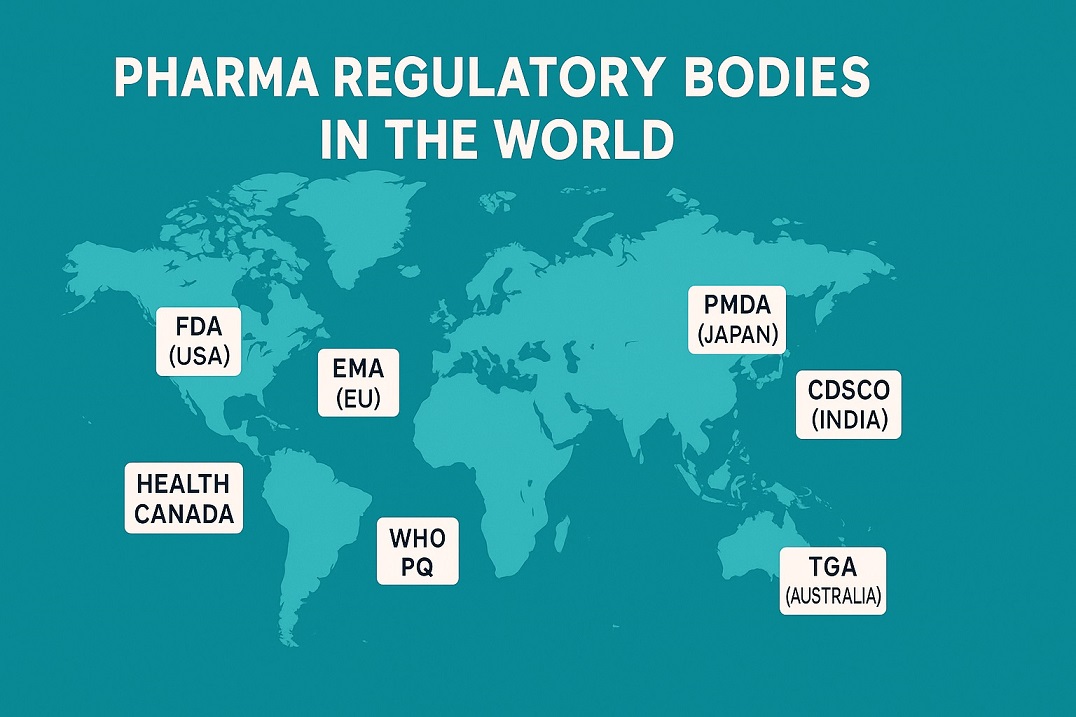
Major Global Pharma Regulatory Bodies
Major pharma regulatory bodies protect medicine quality from lab to pharmacy. They set science-based rules, inspect sites, and review safety data. They also work with ministries, companies, and patients to keep benefits higher than risks.
These agencies share core tasks but work in different legal and cultural systems. They approve medicines, monitor side effects, react to new safety signals, and increasingly align guidelines to support global development.
Major examples include:
United States — FDA (Food and Drug Administration)
Europe — EMA (European Medicines Agency)
United Kingdom — MHRA
Japan — PMDA (Pharmaceuticals and Medical Devices Agency)
India — CDSCO
Canada — Health Canada
Australia — TGA
WHO — Prequalification Programme
The FDA is America’s main drug and food watchdog. Moreover, it reviews safety, quality, and efficacy data before approving new medicines.
The agency helps protect around 330 million people in the United States. It shapes global standards, and many countries follow its major regulatory decisions.
EMA, the European Medicines Agency, helps Europe control medicine safety, quality, and efficacy.It coordinates expert reviews across countries.
Moreover, EMA supports faster access to important treatments.
It shares data and guidance with national regulators.
Centralised assessment of innovative and generic medicines.
Continuous safety monitoring and signal detection.
Scientific advice on development and authorisation plans.
MHRA guides medicine and device safety in the United Kingdom and beyond. Moreover, it carefully reviews trial data and factory inspections before it accepts new products.
Its decisions affect care for over 60 million people and many visitors. MHRA shares guidance globally, shaping standards for other regulators and healthcare teams.
PMDA oversees medicines and devices in Japan to protect public health. Moreover, it reviews data, inspections, and risks before it approves new products.
PMDA supports innovation while it keeps strict standards for safety and efficacy. It also works with global regulators to share findings and improve access for patients worldwide.
Scientific review of drugs and medical devices
Post-marketing safety monitoring and signal detection
Consultation on clinical trial and development plans
CDSCO regulates medicines and devices across India to protect public health. It sets standards for quality, safety, and lab testing.
Moreover, it reviews data, licenses factories, and controls imports for over 1.4 billion people. It works closely with global regulators.
Health Canada carefully regulates medicines and devices to protect people. It sets rules for quality, safety, labeling, and promotion.
Moreover, it reviews data before companies sell new medicines. Its work strongly supports care for about 40 million residents.
TGA oversees medicines and devices across Australia to protect public health. It sets strict rules for quality, safety, and manufacturing standards. Moreover, it reviews evidence before new treatments reach clinics and pharmacies.
The WHO Prequalification Programme checks key medicines, vaccines, and diagnostics. It helps countries without strong regulators find trusted, quality-assured products.
Moreover, it supports donors and agencies when they choose products for health programs. More than 150 countries use prequalified products to protect their populations.
Key Responsibilities of Regulatory Bodies
Regulatory bodies protect people from unsafe or ineffective medicines. They set rules for research, manufacturing, and marketing. Moreover, they guide companies so every step follows standards. Their work links science, ethics, and trust.
A key duty includes clinical trial oversight. Regulators review protocols, consent forms, and safety plans. They track trial progress and handle serious events quickly. Therefore, they confirm data quality before companies request approval. Regulatory submissions play a key role. Companies send structured dossiers with quality, safety, and efficacy data. Regulators assess benefits and risks, then decide if products enter markets. Strong processes support faster access without losing safeguards.
Clinical trial oversight vs no oversight: regulators reduce hidden risks.
Regulatory submissions vs informal reports: agencies demand consistent data.
Global coordination vs isolated work: shared reviews speed safe access.
How Drug Approval Processes Differ Across Regions
Drug approval processes share goals but differ widely across regions. Each authority sets unique rules, timelines, and document formats. Moreover, local disease patterns and budgets shape their review priorities.
Some regulators accept global trial data, others request local confirmatory studies. Therefore, companies adapt strategies, sites, and submissions for every target market. These differences change launch speed, access, and development costs for patients worldwide.
Centralised regional review vs separate national approvals
Priority or fast-track routes vs standard review timelines
Acceptance of global trial data vs demand for local studies
Why Regulatory Knowledge Matters for Pharma Professionals
Regulatory knowledge keeps your daily work safe, legal, and efficient. You understand why rules exist, not just which SOP to follow. Therefore, you design processes that survive inspections and avoid costly mistakes. You prevent delays, failed batches, and compliance findings that hurt patients and your company.
This fluency also boosts your career in QA, RA, PV, and manufacturing. Managers trust people who speak the language of guidelines and dossiers. Moreover, you join key discussions earlier and influence decisions with clear, practical advice. You spot risks in documents and data before they grow. Continuous learning then keeps you ready for new rules and technologies.
Final words
Regulatory bodies work together under global frameworks like pharma regulation to guard patients. Across 194 WHO Member States, agencies share data, align standards, and close safety gaps. Here, we highlight how regulatory bodies ensure drug safety globally.
Today, 36 regulators hold WHO Listed Authority status, showing they meet high quality, safety, and efficacy benchmarks. These trusted agencies support faster access to essential medicines while they keep strict oversight on manufacturing and clinical evidence.
Your career grows when you understand these systems. Stay audit-ready and protect patients by committing to continuing education and structured regulatory learning.
FAQs
1️⃣ What are regulatory bodies in pharma?
Regulatory bodies are official agencies that oversee medicine safety, quality, and efficacy. They set rules, review data, and inspect facilities to protect public health.
2️⃣ Why do regulatory bodies matter for patients?
They help keep unsafe or poor-quality drugs off the market. Their work builds trust, because patients know experts check medicines before and after approval.
3️⃣ How do regulatory bodies work with pharma companies?
They give guidance on studies, submissions, and safety reporting. In return, companies share data and follow rules so new treatments reach patients safely.
References

Ershad Moradi, a Content Marketing Specialist at Zamann Pharma Support, brings 6 years of experience in the pharmaceutical industry. Specializing in pharmaceutical and medical technologies, Ershad is currently focused on expanding his knowledge in marketing and improving communication in the field. Outside of work, Ershad enjoys reading and attending industry related networks to stay up-to-date on the latest advancements. With a passion for continuous learning and growth, Ershad is always looking for new opportunities to enhance his skills and contribute to pharmaceutical industry. Connect with Ershad on Facebook for more information.

Master GxP Validation in 2026: Meaning, Key Steps, and Validated State Control
Auditors want evidence you can trace, not opinions you can explain. GxP validation links intended use, requirements, risk, and test results into one story. When you control changes and review performance, you keep the system inspection-ready every day on time.

Master GMP Compliance in 2026: Meaning, Core Elements, and How to Implement
GMP compliance keeps medicines safe, consistent, and traceable across every batch. This guide explains core GMP elements, practical rollout steps, and common pitfalls. It also shows how to strengthen training, documentation, data integrity, and audit readiness.

History of Pharmacovigilance: From the Thalidomide Crisis (1961–2026) to GMP Oversight
Thalidomide in 1961 changed drug safety forever. Since then, pharmacovigilance has grown from crisis response to proactive risk management. Today, teams track signals, tighten reporting rules, and connect safety data to quality systems. As a result, PV now links directly to GMP oversight, audits, and data integrity.


