A pharmacovigilance internship is now a key doorway into drug safety careers. The global pharmacovigilance market is about USD 9–10 billion in 2025 and could reach USD 16 billion by 2030, with growth near 7–12% annually. At the same time, PV vacancies and outsourcing hubs keep expanding worldwide.
During a PV internship, you practice ICH E2A case handling, E2B(R3) electronic reporting, and E2D–E2E signal management. Entry-level PV specialists often start around USD 65,000–75,000 in the US, while senior roles exceed USD 120,000, making internships a strong launchpad for long-term PV careers for ambitious science graduates.
Table of Contents
What Is a Pharmacovigilance Internship?
A PV internship gives first real experience in drug safety work. Interns help with case intake, data entry, and follow-up calls while learning ICH rules and company procedures. They test if pharmacovigilance matches their strengths and career goals. Interns still study or recently graduated, while trainees already hold a defined role. In PV, internships exist at pharma companies for deep product focus, and at CROs for broader client exposure.
Interns explore the field and build basic pharmacovigilance skills.
Trainees deepen role-specific skills inside one defined position.
CRO internships offer variety and pharma internships offer deeper product focus.
Eligibility & Requirements for a PV Internship
Strong education in pharmacy, pharmacology, or life sciences builds a solid base for a PV or drug safety internship. These science courses help interns understand medicines faster and learn safety case work more easily.
Daily pharmacovigilance tasks also need basic computer skills and medical terminology. Interns use spreadsheets, safety databases, and online tools while reading reports and lab values. Soft skills then connect everything: clear communication, teamwork, time management, and resilience keep work accurate, safe, and efficient under pressure.
Pharmacy degrees focus deeply on medicines; life science degrees cover broader biological systems.
Basic computer skills enable routine tasks; advanced skills accelerate analysis and reporting.
Technical skills show knowledge; soft skills show collaboration, empathy, and communication strength.
Common Requirements
Common Requirements for a PV / Drug Safety Internship – Summary
Degree status: Ongoing or completed degree in pharmacy, medicine, or life sciences; most PV entry roles still request a life-science background.
Communication: Strong written and spoken English for reports and emails; a second language is a plus for global case flows.
Regulatory awareness: Basic knowledge of ICH E2A–E2E and key EMA/FDA rules, as the PV market grows ~7–12% yearly.
Technical: Confident with spreadsheets and safety databases; starter experience with MedDRA or WHO-DD helps a lot.
What You Learn in a Pharmacovigilance Internship
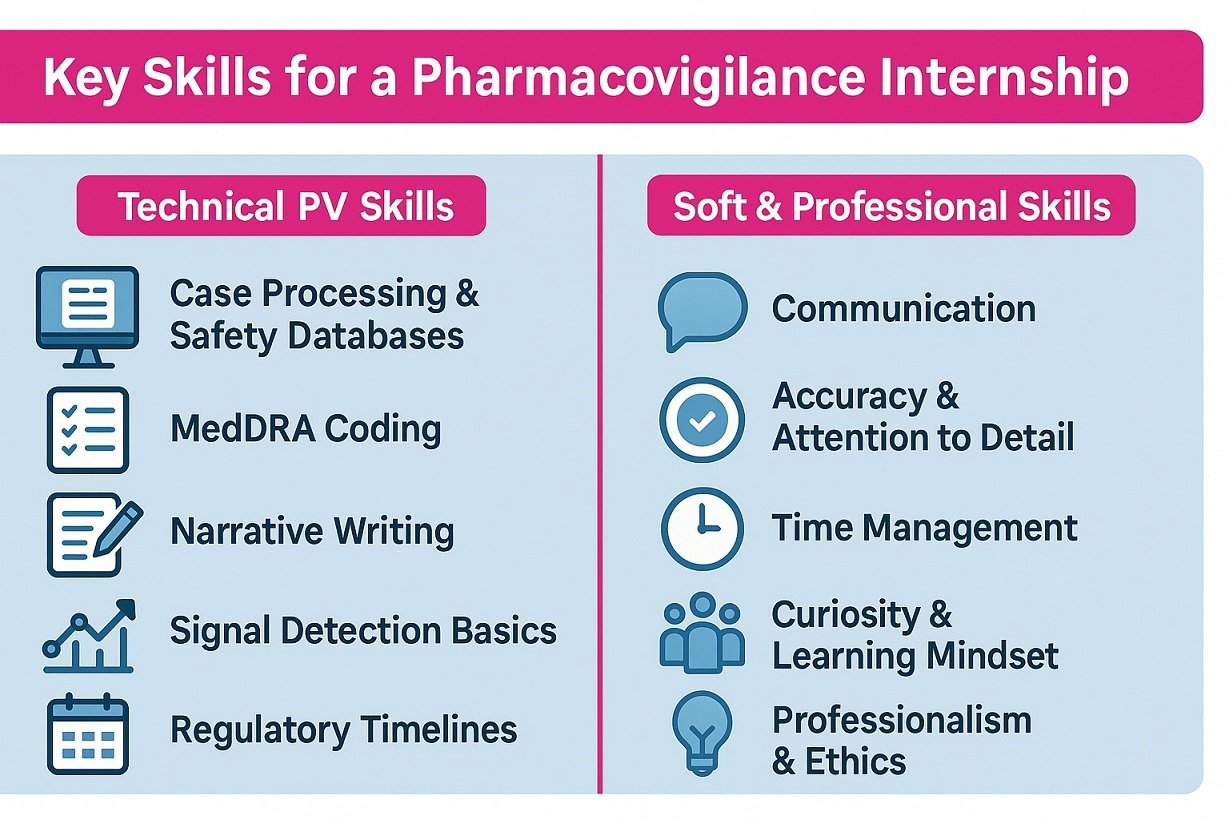
A PV internship builds technical skills in case processing with validated safety databases.
Interns follow ICH E2A 15-day and 90-day regulatory timelines for global safety reports.
Training includes MedDRA coding, narrative writing, and signal detection basics from real case data.
Intern strengths combine technical skills and soft skills: accuracy, communication, time management, teamwork, curiosity, professionalism.
Day-to-Day Responsibilities of a PV Intern
A PV internship introduces interns to practical drug safety work inside real teams. They review simple safety documents, use basic medical terminology, and slowly gain confidence with scientific language and regulations. Supervisors assign structured tasks that match experience, explain goals clearly, and give regular feedback. Interns learn why accuracy and timelines matter, how cross-functional teams protect patients, and they build habits that later support full-time pharmacovigilance roles.
Support literature screening using search terms.
Perform quality checks on safety data.
Provide data entry support in databases.
Help with case triage using rules.
Contribute to compliance tracking for timelines.
How to Find a Pharmacovigilance Internship
Online search engines help candidates find pharma and drug safety roles fast. They scan many sites, highlight fresh vacancies, and use filters for location, experience level, and contract type. Therefore candidates spend less time scrolling and more time applying, while alerts notify them when new pharmacovigilance internship or related roles appear online.
Use clear role keywords in every search.
Combine skill and location phrases when possible.
Set up alerts for “pharmacovigilance” and “GMP” positions.
Specialised platforms, like Pharmuni job board, focus on life science careers. They show roles that match industry skills, certifications, and learning paths. Candidates browse internships, entry roles, and remote options in one place.
The search process feels simpler and more focused than generic engines. Also, links to related courses help candidates close skill gaps early.
Check new listings on Pharmuni job platform daily.
Save interesting roles and compare requirements later.
Follow links to related skill-building courses.
Final words
A pharmacovigilance internship gives first hands-on contact with real safety data. Interns see why EMA tracks about two million adverse reaction reports in EudraVigilance each year. They practice case processing, MedDRA coding, narrative writing, and regulatory timelines on supervised tasks. Many interns move into permanent safety associate roles.
These experiences build core pharmacovigilance skills and show daily teamwork across safety functions. Therefore interns understand EMA expectations and ICH case-reporting standards earlier than many graduates. Additionally they gain confidence using safety databases and speaking clearly about patient risk. To plan next steps, explore Pharmuni pharmacovigilance career path.
FAQs:
1️⃣ Who can apply for a PV intern?
Most programs look for pharmacy, medicine, or life science students and recent graduates. Strong English, basic computer skills, and interest in patient safety also help a lot.
2️⃣ What skills do interns gain during a PV intern?
Interns learn case processing, MedDRA coding basics, narrative writing, and working with safety databases. They also build soft skills like teamwork, communication, and time management under regulatory timelines.
3️⃣ How long does a typical PV intern last?
Most PV internships last 3–6 months, sometimes up to one year. This period gives enough time to learn core tasks and see real safety cycles.
References

Ershad Moradi, a Content Marketing Specialist at Zamann Pharma Support, brings 6 years of experience in the pharmaceutical industry. Specializing in pharmaceutical and medical technologies, Ershad is currently focused on expanding his knowledge in marketing and improving communication in the field. Outside of work, Ershad enjoys reading and attending industry related networks to stay up-to-date on the latest advancements. With a passion for continuous learning and growth, Ershad is always looking for new opportunities to enhance his skills and contribute to pharmaceutical industry. Connect with Ershad on Facebook for more information.

Visual Inspection in Pharma (2026): What It Is, Scope, PDF Guides and SOP Essentials
Visual Inspection checks parenteral units for particles, damage, and labeling errors before release. EU GMP Annex 1 expects inspection of parenteral containers (8.30) and warns visual inspection cannot replace integrity testing, including 100% testing for fusion-sealed volumes ≤100 mL (8.22).

GxP in pharma in 2026: How Inspectors Evaluate System Control
This article explains how GxP quality systems are assessed during inspections, why gaps in governance and execution lead to repeat audit findings, and how regulated pharma operations strengthen inspection readiness through risk management, data integrity, and lifecycle compliance controls.

Quality Oversight In 2026: Roles in pharma industry, GMP Expectations, And Risk-Based Control
Quality Oversight keeps GMP decisions consistent, traceable, and risk-based. It connects deviation handling, CAPA, change control, and trend reviews. It also proves management engagement through documented actions. Strong oversight reduces repeat failures and supports confident batch disposition.


