Hiring has shifted. In 2026, pharma teams hire for skills first, titles second. The phrase “pharma skills for resume” matters because recruiters scan for proof. Meanwhile, six in ten workers need training by 2027, so upskilling wins interviews . And 73% of hiring pros now prioritize skills-based hiring, expanding talent pools up to 10x . Therefore, your pharma skills must be clear, current, and measurable.
In this guide, you’ll identify the most relevant technical and soft skills for pharmaceutical roles and learn how to present them with impact. With references to back each choice smartly.
Table of Contents
Why Skills Matter in Pharma Resumes
Recruiters scan fast. They favor candidates who show the right mix of technical mastery and strong skills for pharma jobs. Therefore, your skills section must be structured, specific, and relevant to the role. You prove fit quickly. You also help the ATS rank you higher.
Moreover, hiring teams use skills to predict impact. They want evidence of quality, safety, and speed. They also check collaboration and decision-making. So, map your skills to outcomes: fewer deviations, smoother audits, faster submissions, and cleaner data.
- Skill-first screening grows talent pools up to 10x on LinkedIn data.
- “Human skills” surged in priority in 2024 among L&D leaders.
- Clear, grouped skills improve ATS parsing and recruiter recall.
- Outcome-tagged bullets raise interview rates in skills-based recruiting.
- Consistent skill labels enable better internal mobility and retention.
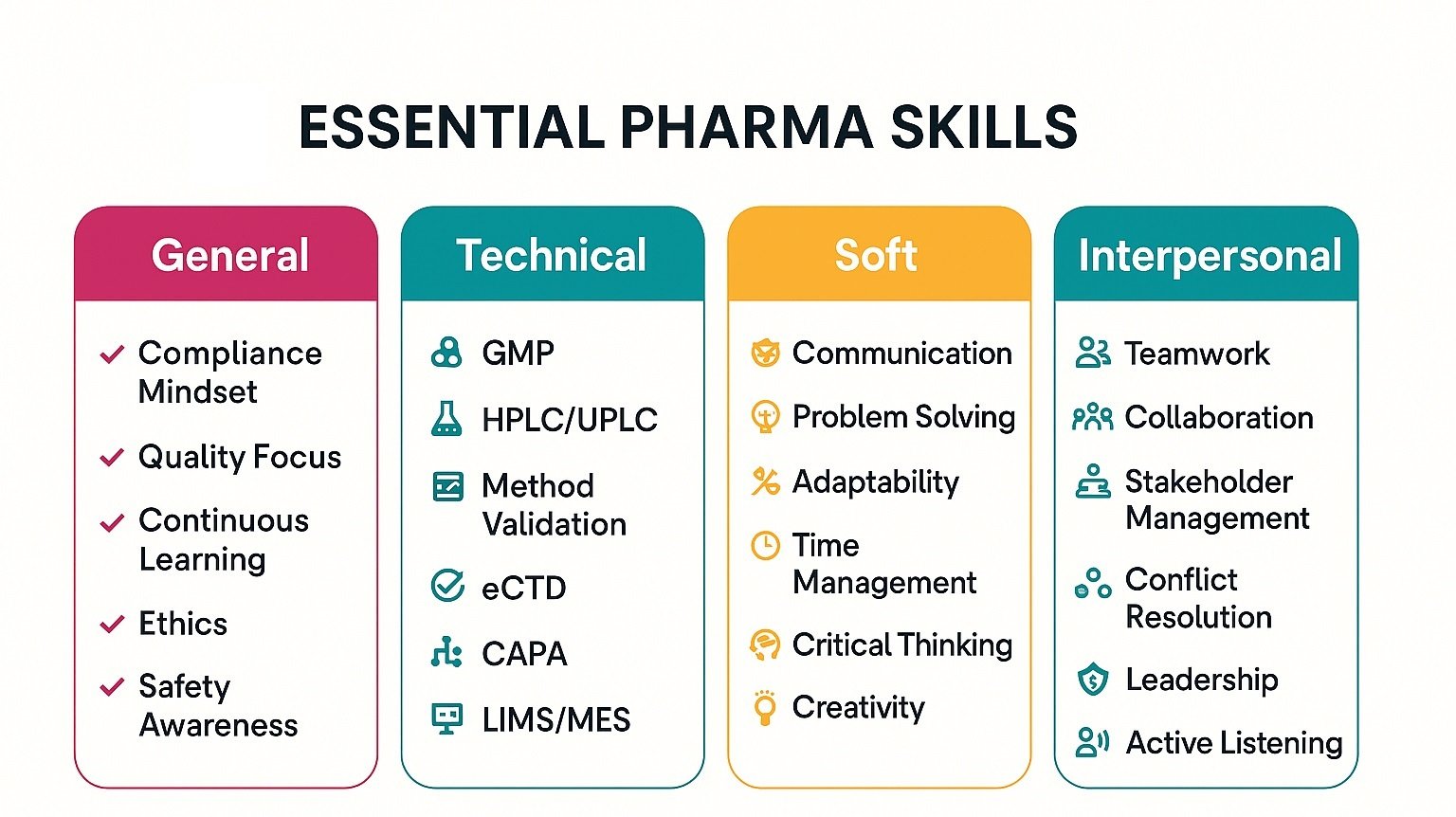
Key Technical Skills for Pharma Resumes
in 2025, employers prioritize technical competencies that prove quality, safety, and speed in environments. Moreover, 73% of recruiters favor skills-based hiring, improving fairness and expanding candidate pools significantly today. Additionally, ATS scores rise when resumes group role-relevant skills using consistent, standardized labels for recruiters.
Next, laboratories value method validation expertise because validated methods cut OOS investigations by double-digits annually. Furthermore, plants reward GMP execution, PPQ strength, and CPV trending that reduce deviation rates measurably. Also, data integrity controls and ALCOA+ practices improve audit readiness and shorten remediation timelines notably.
Then, regulatory literacy across CTD and eCTD accelerates submissions and reduces avoidable authority questions significantly. Likewise, controlled documentation, Part 11 alignment, and Annex 11 knowledge strengthen inspection outcomes and confidence. Moreover, employers value cross-functional fluency with MES, LIMS, ELN, and validated analytical software under GxP. Finally, present these skills with metrics, timelines, and tools to prove immediate impact for employers.
- Quality & Compliance Skills
- Research & Analytical Skills
- Regulatory & Documentation Skills
- Manufacturing & Process Skills
Quality & Compliance Skills
Start with recognized standards to anchor your QMS and compliance story. Then show proven methods that cut defects and cycle time. Finally, prove inspection readiness with clean records, trained teams, and fast CAPA closure. Always tie each item to measurable outcomes.
Standards & Frameworks
- ISO 9001 alignment for consistent QMS execution and audit trails.
- FDA CGMP requirements (21 CFR Parts 210/211) for drug quality.
- ICH Q9 (Quality Risk Management) and ICH Q10 (Pharma Quality System).
Methods & Tools
- CAPA and deviation management with root-cause depth (e.g., 5-Why, Fishbone).
- Six Sigma (DMAIC: 5 steps) for variation control and yield.
- Risk assessment and control plans tied to change control.
Audit & Inspection Readiness
- SOP lifecycle, GxP training records, and data integrity governance.
- Mock inspections, KQIs/KPIs dashboards, and corrective action closure speed.
- Supplier quality oversight and material traceability.
Research & Analytical Skills
Follow GLP and document every step. For example, keep bound notebooks with signed witness lines. Then run QC release tests and trend results weekly. For example, cut assay OOS from 3% to 1% in Q2. Next, develop an HPLC method for your API at 254 nm. Also, meet system suitability targets: Rs > 2.0 and tailing < 1.5. Moreover, prove robustness across ±10% flow and temperature shifts.
Validate methods thoroughly and show hard numbers. For example, report accuracy 99–101% and precision RSD < 2%. Then execute stability per ICH Q1A to justify shelf life. For example, accelerated 40°C/75%RH supported 24-month claims. Next, use DoE to optimize gradient and pH. For example, cut runtime 30% and solvent 25%. Finally, lead root-cause analysis and fix issues fast. For example, replace leaking pump seals and close OOS in five days.
Regulatory & Documentation Skills
Regulatory roles need sharp documentation skills. Think Regulatory Affairs Associate, CMC Specialist, Labeling Manager, Pharmacovigilance Officer, RIM Specialist, Documentation Coordinator, and Submission Manager. Use the following to deliver compliant files fast and reduce rework.
Dossier authoring: CTD/eCTD modules, summaries, and granularity
Labeling control: variation/CMC changes, and lifecycle submissions
Pharmacovigilance basics: signal awareness and safety reporting flows
Controlled vocabularies: metadata, and versioning discipline
Document control: templates, review cycles, and audit trails
Traceability: requirements ↔ evidence mapping and inspection packs
Manufacturing & Process Skills
Strong pharma manufacturing skills turn procedures into reliable batches. Moreover, they protect patients and margins. Therefore, show proof of control, cleanliness, and uptime. Also, quantify rework cuts, yield gains, and faster releases. Finally, link every tool to a measurable outcome.
Why each item matters
- GMP operations and aseptic practices; gowning and contamination control.
- Batch manufacturing records (BMR/BPR) with right-first-time focus.
- Process validation and PPQ; CPV trending for sustained state.
- Equipment qualification (IQ/OQ/PQ) and maintenance planning.
- Cleaning validation; worst-case matrices and carryover limits.
- Tech transfer: receiving site readiness, comparability, and scale-up risk.
- MES/EQMS/LIMS familiarity and deviation-to-CAPA integration.
How to Present Pharma Skills on Your Resume
Group your pharma skills under clear, scannable sections. Use categories like Analytical, Quality/Compliance, Regulatory, and Manufacturing. Place role-relevant skills near the top. Mirror keywords from the job description for ATS alignment. Also add tools, systems, and standards beside each skill. Moreover, quantify results using deviations reduced, cycle time improved, or yield increased. Additionally, include certifications that validate methods or systems. Therefore, use the Pharmuni Resume Builder to format and refine quickly.
Balance technical mastery with interpersonal strengths that enable regulated teamwork. Show communication, collaboration, and problem solving with measurable outcomes. Embed prioritized skills within experience bullets using numbers and timelines. Also integrate role keywords into bullets, headings, and summaries. Meanwhile, refresh skills for each application and remove weak items.

Final words
Skill-driven resumes matter now more than ever. FDA’s FY2024 quality report logged 421 drug recalls, the lowest in five years; contamination was the most common defect. Meanwhile, domestic warning letters fell from 59 to 41, but foreign letters rose from 35 to 64. These signals highlight skills that prevent contamination, sustain CGMP control, and tighten documentation. Therefore, show competencies that reduce deviations, strengthen investigations, and support clean inspections.
Recruiters also value speed and rigor after inspections. FDA expects firms to answer a Form 483 within 15 business days with credible corrective actions. So, spotlight capabilities in CAPA, change control, data integrity, and aseptic practices. Then map each skill to outcomes like faster closure and fewer repeats. Finally, upskill with Pharmuni’s role-aligned courses, and convert learning into proof using the Pharma Resume Builder. That way, your resume links skills to FDA-relevant results recruiters trust.
FAQs:
1️⃣ What top pharma skills impress recruiters now?
List GMP, method validation, HPLC/UPLC, eCTD, CAPA, data integrity, LIMS/MES. Also add communication, teamwork, problem solving, and attention to detail.
2️⃣ How many skills should I include?
Show 10–16 skills grouped by category. Then order by relevance to the role.
3️⃣ How can I prove these skills quickly?
Use metrics, tools, and certifications with each skill. For example, “Reduced deviations 23%” or “Validated six HPLC methods.”
Refrences

Ershad Moradi, a Content Marketing Specialist at Zamann Pharma Support, brings 6 years of experience in the pharmaceutical industry. Specializing in pharmaceutical and medical technologies, Ershad is currently focused on expanding his knowledge in marketing and improving communication in the field. Outside of work, Ershad enjoys reading and attending industry related networks to stay up-to-date on the latest advancements. With a passion for continuous learning and growth, Ershad is always looking for new opportunities to enhance his skills and contribute to pharmaceutical industry. Connect with Ershad on Facebook for more information.

Revalidation In Pharma (2026 guide): Meaning, Triggers, Frequency, And Requalification Differences
Revalidation protects product quality and business continuity by linking GMP decisions to risk, evidence, and context. This article helps teams decide when to act, what to document, and how to classify issues consistently before audits and regulatory inspections.

GMP Regulations in 2026: How Inspectors Assess Compliance and Control Systems
This article explains how pharmaceutical regulatory requirements shape inspection decisions, why GMP compliance gaps persist across manufacturing sites, and which operational controls, documentation practices, and risk-based measures support inspection readiness under global GMP standards.

WHO GMP in 2026: Inspection Readiness and Compliance Expectations
This article explains how global pharmaceutical GMP standards are applied during inspections, why operational gaps persist despite formal compliance, and how quality systems, contamination control, and risk-based execution shape regulatory inspection readiness across manufacturing operations.


