Short-term and certification-based programs are reshaping pharma learning. If you’re checking pharma courses eligibility, micro-credentials let professionals and students upskill fast, stack credits, and signal job-ready skills. In fact, 96% of employers say micro-credentials strengthen a candidate’s application.
Modern Pharma Courses are flexible, online, and stackable—perfect alongside or before diplomas and degrees. Healthcare projects about 1.9 million openings per year from 2024–2034, so targeted GMP, QA, and PV certificates map tightly to hiring demand.
After reading this article, you’ll clearly understand eligibility, entry requirements, and benefits of pharma certification programs. You’ll also get a brief, practical comparison with diploma, bachelor’s, and master’s courses for context.
Table of Contents
Eligibility Overview for Degree and Diploma Programs
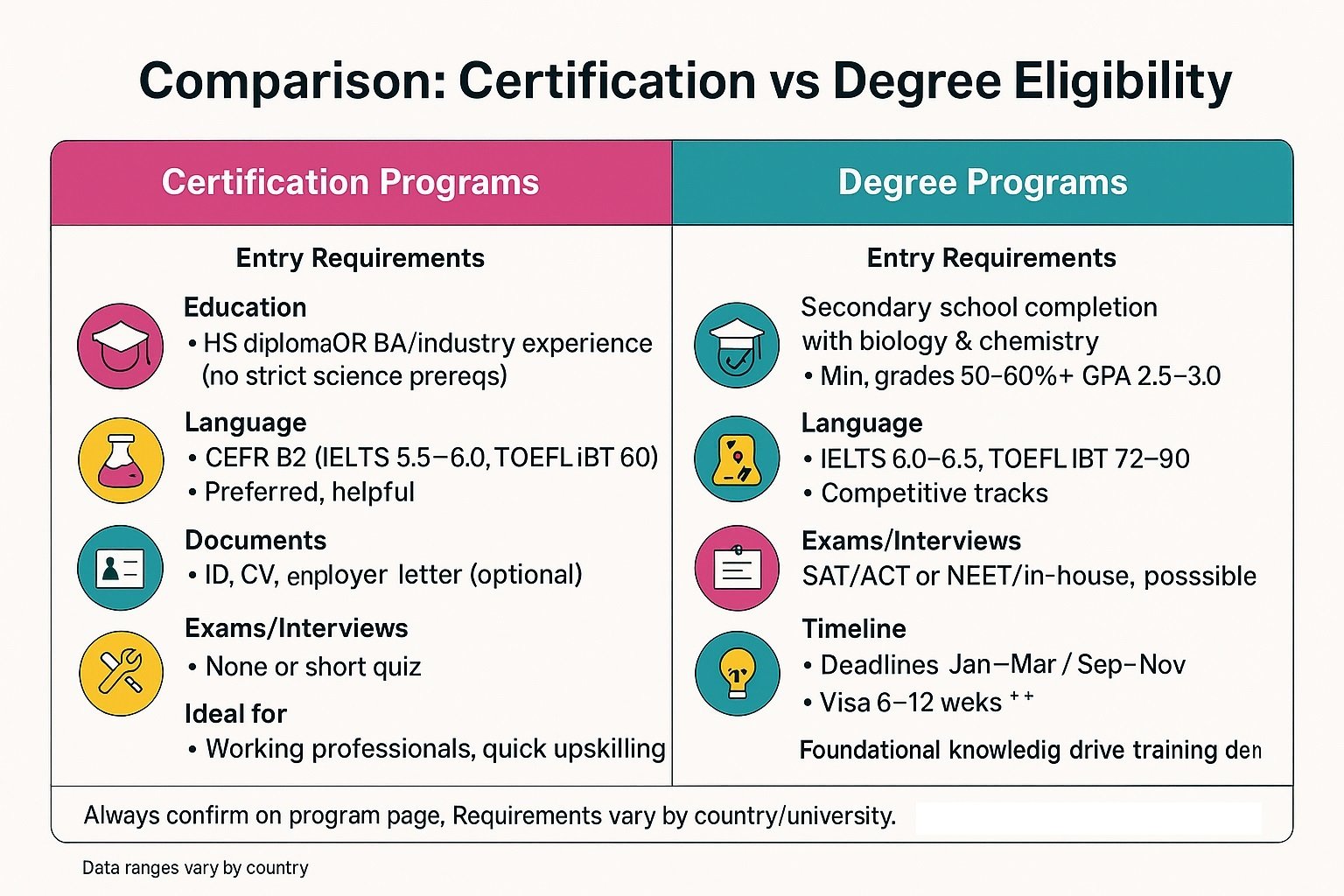
Diplomas and bachelor’s programs: Most diplomas require secondary school with biology/chemistry and 50–60%+ in science (or GPA 2.5–3.0). Some schools accept vocational equivalents. First, check each pharma course’s requirements and language tests—IELTS 6.0–6.5, TOEFL iBT 72–90, or PTE 55–58. Universities may add entrance exams (SAT/ACT, NEET, or in-house) or brief interviews. Competitive tracks prefer 20–40 lab hours.
International applicants verify visa timelines (6–12 weeks) and certified translations. Always confirm deadlines (Jan–Mar / Sep–Nov) and required documents.
Diploma: secondary school, science subjects, language proof
Bachelor’s: strong GPA, exams, science background
Master’s: relevant degree, GPA, tests, experience
Always confirm each school’s pharma courses requirement.
Why Pharma Certification Courses Are in Demand?
Pharma certification demand is rising as teams need fast, targeted upskilling. Roles shift with new rules and tech: EMA’s Annex 1 (2022) mandates a site-wide Contamination Control Strategy; ICH Q9(R1), effective 26 July 2023 in the EU, elevates risk-based decision-making; FDA’s data-integrity guidance sharpens ALCOA+ and lifecycle controls. Employers want proof of applied competence, so programs teach daily execution—SOPs, CAPA, inspection readiness, submissions, audits, and defensible record review—without forcing professionals to pause work.
Flexible schedules, virtual classrooms, and online labs support distributed teams and cross-border projects. Stackable micro-credentials build toward diplomas or degrees, signaling momentum. Employers reward current knowledge in GMP, GCP, and submission workflows with interviews and promotions. Persistent FDA Form 483 patterns keep pressure on procedures and training, reinforcing root-cause thinking and right-first-time delivery. Certificates also enable shifts into QA, pharmacovigilance, or clinical operations, aligning skills precisely to job descriptions.
Eligibility Criteria for Pharma Certification Courses
Pharma certification courses set clear eligibility. Most programs accept science or allied backgrounds. Many include pharmacy, biotech, life science, or healthcare diplomas. Programs request basic computer skills and reliable internet. Providers test English or local language for comprehension. Therefore, review course pages for specific prerequisites.
Freshers — Eligibility and Fit
Freshers build foundations and show motivation. They bring school science or a recent diploma. They value clear entry steps and supportive mentors.
Show science subjects and curiosity
Pass a readiness quiz or orientation
Use beginner labs or simulations
Write simple SOPs and reports
Working Professionals — Eligibility and Fit
Working professionals target impact and clearer roles. They present experience in QA, regulatory, or clinical. Additionally, they align learning with performance goals. They demonstrate English or local language for reporting.
Provide one to three years relevant experience
Match certificates to role outcomes
Share recent projects with measurable results
Academic and Professional Background
Graduates & freshers: Pharmacy, life sciences, chemistry (plus biotech, microbiology, biochemistry, allied health, biomedical engineering) are accepted. Check subjects/GPA/docs. Start with foundation modules if needed. Programs value lab literacy, scientific writing, computer/Internet access, and language proficiency. Some require a readiness quiz or short interview; providers publish clear eligibility rubrics.
Working professionals: Qualify via QA/RA/clinical experience. Submit project summaries, audit exposure, and measurable improvements. Use bridge modules to close gaps and pick the right level per course requirements. Managers can target leadership-focused certificates.
Language & Technical Requirements
Online pharma programs expect clear English communication for global teamwork. Learners read protocols, write reports, and discuss findings in English. Moreover, providers ask for basic digital literacy and reliable internet access. Students navigate LMS portals, spreadsheets, and video platforms like Zoom. Programs may request IELTS, TOEFL, or CEFR scores for placement. Some offer language support modules for technical vocabulary and writing.
Courses align with global standards to improve transferability and compliance. Therefore, many programs reference ICH, WHO, and regional GMP frameworks. They also include computer validation, data integrity, and documentation control basics. Certificates often map competencies to roles in QA, PV, and RA.
ICH E6(R2)/(R3) GCP for clinical research quality and ethics
ISO 9001, ISO 13485 for quality management systems and device processes
FDA 21 CFR Part 11, EU Annex 11 for electronic records
ISO 17025 for laboratory competence and method validation
WHO GMP for manufacturing practices across global markets
Final words
Certification courses now drive faster pharma career growth. Compliance scrutiny keeps rising, with 105 FDA warning letters to human drug manufacturers in FY2024— the highest in five years. U.S. Food and Drug Administration Meanwhile, science roles keep expanding: medical scientists are projected to grow 9% from 2024–2034, supporting steady demand for current, verifiable skills.
Choose a focused path, then stack credentials that prove outcomes. Employers increasingly act on skills signals—87% hired at least one micro-credential holder in the past year—so timely certificates help you stand out and advance
FAQs:
1️⃣ Who is eligible for pharma certification programs?
Most programs welcome graduates in pharmacy, life sciences, chemistry, or related fields. Freshers start with foundations; working professionals target role-focused tracks. Check each school’s pharma courses eligibility page for subject and GPA notes.
2️⃣ What are the typical requirements for pharma courses?
Review each pharma courses requirement before applying. Providers usually ask for transcripts, ID, and language proof. You also need basic digital skills and reliable internet.
3️⃣ What benefits do certifications provide?
Certificates help you upskill quickly and map to job tasks. They stack toward diplomas or degrees and strengthen applications.
Refrences

Ershad Moradi, a Content Marketing Specialist at Zamann Pharma Support, brings 6 years of experience in the pharmaceutical industry. Specializing in pharmaceutical and medical technologies, Ershad is currently focused on expanding his knowledge in marketing and improving communication in the field. Outside of work, Ershad enjoys reading and attending industry related networks to stay up-to-date on the latest advancements. With a passion for continuous learning and growth, Ershad is always looking for new opportunities to enhance his skills and contribute to pharmaceutical industry. Connect with Ershad on Facebook for more information.

Revalidation In Pharma (2026 guide): Meaning, Triggers, Frequency, And Requalification Differences
Revalidation protects product quality and business continuity by linking GMP decisions to risk, evidence, and context. This article helps teams decide when to act, what to document, and how to classify issues consistently before audits and regulatory inspections.

GMP Regulations in 2026: How Inspectors Assess Compliance and Control Systems
This article explains how pharmaceutical regulatory requirements shape inspection decisions, why GMP compliance gaps persist across manufacturing sites, and which operational controls, documentation practices, and risk-based measures support inspection readiness under global GMP standards.

WHO GMP in 2026: Inspection Readiness and Compliance Expectations
This article explains how global pharmaceutical GMP standards are applied during inspections, why operational gaps persist despite formal compliance, and how quality systems, contamination control, and risk-based execution shape regulatory inspection readiness across manufacturing operations.


