Patients trust medicines to work every time. The GMP vs cGMP difference matters because quality failures still occur. According to WHO, at least 1 in 10 medicines in low- and middle-income countries are substandard or falsified. This reality raises risk, wastes budgets, and harms people.
This reality raises risk, wastes budgets, and harms people. Therefore, manufacturers and regulators rely on Good Manufacturing Practice (GMP) and current Good Manufacturing Practice (cGMP) to protect quality. Together, they create a living system that prevents errors, enables control, and proves compliance. In this guide, you will learn what GMP vs cGMP means, how they differ, and why the “current” matters in 2026 and beyond.
What Is GMP (Good Manufacturing Practice)?
GMP sets the minimum standards for making, controlling, and releasing medicines. In the EU, the European Medicines Agency defines GMP as the minimum standard manufacturers must meet during production. Inspectors verify compliance across the network.
Practically, GMP covers people, premises, processes, and proof. It defines who does each task, where they do it, how they do it, and which records show it happened. Therefore, GMP requires robust SOPs, validated methods, qualified equipment, controlled environments, calibrated instruments, and trained teams. Moreover, quality units must review data and authorize release. Finally, change control, CAPA, audits, and supplier oversight keep the system healthy.
Global regulators share the same goal. EMA, FDA, and WHO all push consistent, safe, and effective products. However, each region structures rules and guidance differently. Consequently, teams must map site procedures to local law while honoring universal GMP principles.
What Does “cGMP” Mean?
cGMP adds one critical letter: the “c.” The FDA explains it plainly: the “C” in CGMP stands for current, requiring companies to use up-to-date technologies and systems.
In other words, cGMP does not let you freeze processes in time. Instead, it demands continuous improvement and modern controls. As new risks emerge, methods evolve. As tools advance, expectations rise. Therefore, quality systems must keep pace.
The FDA’s cGMP regulations also describe minimum requirements for facilities, methods, and controls used in manufacturing, processing, and packing drug products. The goal is simple: ensure identity, strength, quality, and purity for every batch. Consequently, firms must maintain a state of control, use data to guide decisions, and upgrade procedures when better science or technology appears.
Key Differences Explained
Both concepts protect patients. However, GMP and cGMP differ in emphasis and behavior. Use this side-by-side view to clarify the gap:
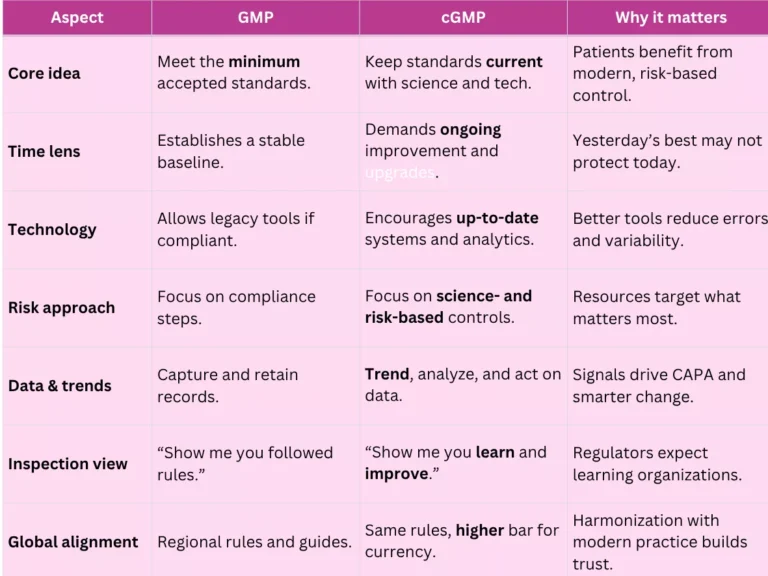
GMP sets the floor; cGMP raises the ceiling—every year. Therefore, teams should adopt modern validation concepts, electronic batch records, data integrity by design, digital QMS, and ongoing training. In practice, these choices reduce deviations, speed investigations, and strengthen inspection readiness.
Common Misconceptions
Even experienced teams mix things up here. Jargon blurs basics, and myths spread fast. Before your next audit or SOP update, skim these quick clarifications—they’ll save time, rework, and stress.
- “GMP vs cGMP are different laws.” Not quite. cGMP reflects the same legal framework but emphasizes up-to-date execution, especially in FDA contexts.
- “GMP is outdated.” Incorrect. GMP remains the foundation. However, cGMP reminds teams to modernize tools and methods as standards evolve.
- “If we comply once, we stay compliant.” Compliance drifts. Therefore, you must maintain systems, trend data, and upgrade controls proactively.
- “Only FDA uses cGMP thinking.” The language differs across regions, yet regulators worldwide push continuous improvement and risk-based quality systems. EMA defines GMP as minimum standards but supports harmonization and modern oversight.
Master GMP. Pass audits with confidence.
Why the “Current” Matters in 2026
The threat landscape keeps shifting. Substandard and falsified products still circulate globally, and WHO continues to highlight the harm and cost.
Therefore, manufacturers must tighten controls, verify supply chains, and strengthen data integrity. Moreover, digital manufacturing, PAT, and advanced analytics now help teams detect drift earlier and respond faster. As a result, companies that embrace cGMP thinking reduce rework, cut complaints, and pass inspections with fewer observations.
How to Move From “GMP-only” to “cGMP Always”
Use these practical actions to embed lessons into daily work:
- Map your gaps: Compare SOPs, validation, and data integrity controls against current guidance and inspection trends.
- Prioritize risk: Focus first on products, processes, and suppliers with the highest patient impact.
- Modernize tools: Evaluate electronic batch records, validated QMS, and automated trend dashboards.
- Strengthen training: Teach teams why steps exist, not just what to do.
- Trend relentlessly: Monitor yields, deviations, OOS/OOTs, complaints, and audit findings. Then act.
- Refresh validation: Apply lifecycle thinking to facilities, utilities, equipment, and computerized systems.
Final words
GMP builds the foundation. cGMP keeps it strong and current. Together, they protect identity, strength, quality, and purity for every batch. The FDA stresses the “current” expectation, while EMA defines the minimum standards and coordinates inspections across the EU.
Meanwhile, WHO’s data reinforces why quality systems matter far beyond audits: poor-quality medicines still harm patients and drain health budgets.
Therefore, treat GMP vs cGMP as two sides of one commitment. Build robust baselines, then keep improving. Invest in people, modernize systems, and let data drive smarter control. Finally, align global frameworks, maintain inspection readiness, and protect patients first—every time.
FAQ
GMP sets minimum manufacturing standards. cGMP adds current expectations. Therefore, you must keep systems up to date. Both protect product quality and safety.
GMP defines the baseline. However, cGMP requires continuous improvement using modern science and technology.
The FDA uses “cGMP” wording. EMA and WHO reference GMP. Yet all expect robust, modern manufacturing standards and regulatory compliance.
References:

Stephanie Männicke
Digital Marketing Especialist at Zamann Pharma Support, brings 8 years of experience in Corporate and Digital Communication. Specializing in Digital Marketing and Content Creation, Stephanie is currently focused on creating strategic content for Pharmuni's networks, especially content on topics such as recruitment, onboarding and employer branding. Outside of work, Stephanie is a mum, a crocheter and a movie fan. An avid reader and in search of expanding her knowledge, Stephanie is always looking for ways to innovate communication in the digital environment and connect people in a genuine way.

Revalidation In Pharma (2026 guide): Meaning, Triggers, Frequency, And Requalification Differences
Revalidation protects product quality and business continuity by linking GMP decisions to risk, evidence, and context. This article helps teams decide when to act, what

GMP Regulations in 2026: How Inspectors Assess Compliance and Control Systems
This article explains how pharmaceutical regulatory requirements shape inspection decisions, why GMP compliance gaps persist across manufacturing sites, and which operational controls, documentation practices, and

WHO GMP in 2026: Inspection Readiness and Compliance Expectations
This article explains how global pharmaceutical GMP standards are applied during inspections, why operational gaps persist despite formal compliance, and how quality systems, contamination control,


