Pharma validation jobs keep medicines safe and consistent. Demand stays strong as pipelines grow and factories modernize. For example, thousands of “validation engineer” openings appear across major markets each month. Moreover, US validation engineers report six-figure averages, while Europe shows healthy mid-career ranges. Life-sciences capital expenditures grew ~13% per year (2022–2024), as companies built biologics capacity and modernized digital systems that require CSV.
Therefore, this guide helps you explore roles, must-have skills, and smarter search tactics. Additionally, you will learn salary ranges and realistic growth paths so you plan your next move with confidence. Finally, you get a visual workflow to find the right pharma jobs faster on our portal.
What Are Validation Jobs in the Pharmaceutical Industry?
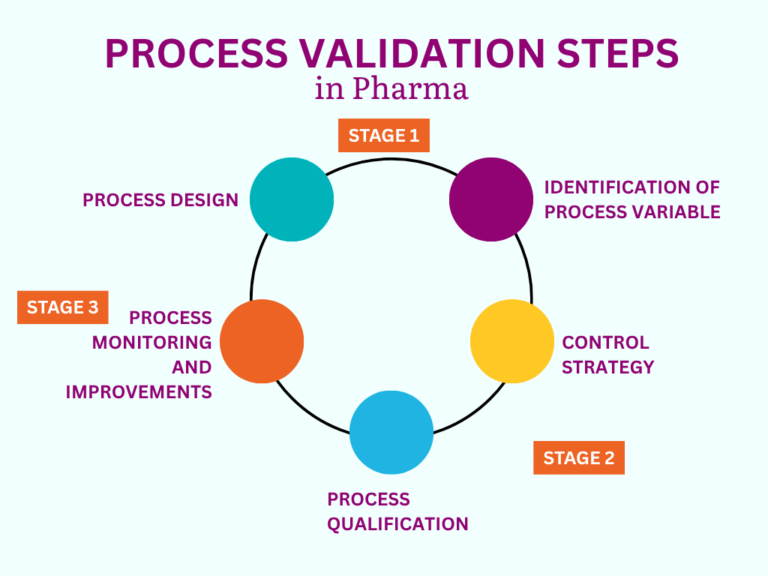
Validation proves processes, equipment, cleaning, and computerized systems work as intended. Teams design protocols, execute tests, analyze data, and approve results. Then they lock in control so products meet quality, safety, and efficacy standards—every batch, every time.
Types of Pharmaceutical Validation Jobs
Use this quick map of core roles, plus one practical example per type.
- Process Validation (PV): Confirms a manufacturing process delivers consistent, compliant quality. Example: Run three PPQ lots on a sterile fill line after changes.
- Equipment / Facility Validation (IQ/OQ/PQ): Engineers qualify machines, utilities, and cleanrooms to defined specs. Example: Commission and qualify a new autoclave and clean steam loop.
- Cleaning Validation (CV): Evidence shows cleaning removes residues below safe carryover limits. Example: Demonstrate CIP keeps API levels under MACO for shared tanks.
- Computer System Validation (CSV): Teams validate GxP software and data integrity controls per GAMP 5. Example: Qualify LIMS/eQMS to comply with 21 CFR Part 11 and Annex 11.
These tracks often overlap on big tech-transfer or expansion projects. However, picking one focus first helps you specialize and advance faster.
How to Find Validation Engineer Positions in Pharma
Follow this step-by-step flow to search smarter on our jobs portal. Keep it simple. Move fast. Iterate quickly.
- Search & filter smartly. Type “validation” (plus CSV, PPQ, IQ/OQ/PQ) and filter by job type, seniority, location, and remote/hybrid.
- Refine with skills keywords. Add GAMP 5, Annex 15, 21 CFR Part 11, LIMS/MES/eQMS to surface relevant roles.
- Shortlist fast. Open role cards, aim for a 70%+ skills match, and save the best fits.
- Tailor & apply. Mirror keywords, quantify wins (“reduced residues 20%”), and track submissions and follow-ups.
- Repeat weekly. Re-run the same filters twice a week to catch fresh postings.
Pharma Validation Salary Insights and Career Progression
Salaries vary by region, seniority, and site complexity. However, recent benchmarks show clear patterns:
- United States: Validation engineers average around $125k annually. Senior roles can reach $158k+, with higher figures in biotech hubs.
- Germany: A typical validation engineer earns €60k on average, with higher pay in major metros.
- United Kingdom (CSV focus): CSV roles average about £35k, yet specialized or lead posts pay more.
- India: Validation engineer averages cluster near ₹6.5–7.5 LPA, with top earners much higher in pharma hubs.
Openings remain plentiful in mature markets. For instance, US platforms list many thousands of process validation roles at any time, reflecting constant GMP hiring cycles.
What drives pay?
Experience with high-risk processes, sterile operations, data integrity, and high-throughput biologics often lifts compensation. Moreover, greenfield builds, major tech transfers, and remediation programs command premiums. In addition, regulated software work (CSV, Annex 11, Part 11) remains a strong differentiator.
Market outlook
Quality and validation hiring stays resilient across EU and the UK, as companies prepare launches and navigate evolving compliance. Consequently, demand for validation and regulatory expertise remains among the highest in life sciences hiring lists.
Career progression paths
- From Validation Specialist → Senior Validation Engineer → Validation Lead/Manager: Own strategy, risk prioritization, and cross-site harmonization in pharma validation jobs.
- For digital tracks (CSV): CSV Analyst → CSV Lead → Head of Computerized Systems Compliance; govern data integrity, audits, and eQMS/MES programs.
- Process-focused professionals: Advance into QA Manager or QP support, bridging shop-floor control, QMS leadership, and release decisions.
- Experienced leaders: Pivot to consulting as a Regulatory Consultant or Remediation Program Manager for faster pay growth and broader project variety.
Final Words
You saw what validation means, which roles exist, and how to search. Moreover, you learned salary ranges, growth drivers, and proven advancement paths.
Demand looks real, not hype. In the U.S., validation engineers average ~$125k, while senior specialists can exceed $158k in major biotech hubs. In Europe, Germany centers near €60k, and UK CSV roles commonly span £35–60k. Meanwhile, India’s experienced validation engineers often earn ₹7–12 LPA, with higher bands in pharma hubs.
Therefore, take action now. Shortlist target roles. Then tailor your resume with quantified wins and relevant keywords. Finally, apply in focused batches and track every follow-up.
FAQs
Validation jobs in pharmaceutical industry confirm processes, equipment, cleaning, and systems work consistently. Moreover, they protect product quality and patient safety under GMP rules.
Process Validation, Equipment/Facility Qualification, Cleaning Validation, and Computer System Validation. Additionally, CSV demand rises with data integrity and digital systems.
Prioritize IQ/OQ/PQ, PPQ, risk-based thinking, and GAMP 5. Furthermore, know Annex 15, 21 CFR 11/210/211, and deviation/CAPA.
Pay varies by region, seniority, and site complexity. However, specialized sterile, biologics, or CSV work typically commands higher bands.
References:

Stephanie Männicke
Digital Marketing Especialist at Zamann Pharma Support, brings 8 years of experience in Corporate and Digital Communication. Specializing in Digital Marketing and Content Creation, Stephanie is currently focused on creating strategic content for Pharmuni's networks, especially content on topics such as recruitment, onboarding and employer branding. Outside of work, Stephanie is a mum, a crocheter and a movie fan. An avid reader and in search of expanding her knowledge, Stephanie is always looking for ways to innovate communication in the digital environment and connect people in a genuine way.

GxP in pharma in 2026: How Inspectors Evaluate System Control
This article explains how GxP quality systems are assessed during inspections, why gaps in governance and execution lead to repeat audit findings, and how regulated

Quality Oversight In 2026: Roles in pharma industry, GMP Expectations, And Risk-Based Control
Quality Oversight keeps GMP decisions consistent, traceable, and risk-based. It connects deviation handling, CAPA, change control, and trend reviews. It also proves management engagement through

Revalidation In Pharma (2026 guide): Meaning, Triggers, Frequency, And Requalification Differences
Revalidation protects product quality and business continuity by linking GMP decisions to risk, evidence, and context. This article helps teams decide when to act, what