Pharma Production must protect patients with repeatable, controlled manufacturing. WHO warns that at least 1 in 10 medicines in low- and middle-income countries are substandard or falsified, costing about US$30.5 billion each year—so robust GMP controls matter everywhere today.
Regulators set the baseline: EU GMP Annex 1 was published 25 Aug 2022 and became effective 25 Aug 2023, shaping sterile manufacturing expectations. In the US, 21 CFR 211 sets minimum cGMP for drug products. Here’s the simple map: roles → requirements → sterile basics → process steps, from QA/QC and engineering through aseptic controls, batch records, and release
Table of Contents
What is Pharma Production
Pharma Production makes medicines in controlled, documented steps. Teams follow GMP to protect patients and product quality. They control materials, equipment, and cleanroom behavior every day. They record each action in batch documents and logs. Therefore, strong controls reduce errors and speed safe batch release.
It turns approved formulas into finished doses, like tablets or sterile vials.
It uses SOPs, in-process checks, and deviation handling to prevent defects.
It links QA, QC, engineering, and operators to keep processes stable.
Key Roles in Pharma Industry — Manufacturing, QA, QC, Engineering
Pharma manufacturing runs on clear roles and shared accountability. Each team controls risk and keeps batches consistent. They follow SOPs and document every critical action. Therefore, strong teamwork prevents delays and protects patients.
Manufacturing Operator: runs equipment, follows batch records, and reports deviations fast.
Quality Assurance (QA): reviews records, approves changes, and decides batch disposition.
Quality Control (QC): tests raw materials and products, and verifies results against specs.
Engineering: maintains utilities and machines, and supports calibration and troubleshooting.
Pharma Manufacturing and Production Meaning, Scope, and Key Terms
Pharma manufacturing means making medicines through controlled, documented steps. It covers people, processes, equipment, utilities, and quality checks. Teams follow GMP to prevent mix-ups, contamination, and errors. So, production links development plans to reliable commercial supply.
- Where Pharma Manufacturing Fits in the Product Lifecycle
- API Production vs Finished Drug Product Manufacturing
- Batch manufacturing vs continuous Manufacturing
Where Pharma Manufacturing Fits in the Product Lifecycle
Manufacturing starts after teams lock the process and controls. It delivers consistent batches for patients and markets. It also supports scale-up and tech transfer.
It converts development knowledge into routine manufacturing steps.
It uses validated methods and defined acceptance criteria.
It controls materials, equipment, and cleanroom behavior daily.
It generates batch records for QA review and release decisions.
It feeds trends back into improvements and lifecycle updates.
API Production vs Finished Drug Product Manufacturing
API manufacturing creates the active substance that drives the medicine’s effect. Teams control reactions, purification, and drying to meet specs. They manage impurities, yield, and raw material quality.
Finished drug product manufacturing makes the final dosage form. Teams blend, granulate, compress, coat, or fill and seal units. They control uniformity, sterility where needed, and packaging integrity.
Both stages use GMP and tight documentation. They link through validated transfers and clear acceptance criteria.
Batch manufacturing vs continuous Manufacturing
Batch manufacturing makes medicine in defined lots with start and end points. Teams run a recipe, collect samples, and review batch records. They release each lot after QA review and testing.
Continuous manufacturing runs material through the process without long stops. Teams control quality with sensors, trends, and real-time adjustments. They focus on steady state and rapid detection of drift.
Batch fits flexible products, smaller volumes, and frequent changeovers.
Continuous fits high volume, stable demand, and strong process control maturity.
Pharma Production Requirements — EU GMP vs FDA 21 CFR 211
EU GMP sets expectations for quality systems, documentation, trained staff, and controlled manufacturing every day.
FDA 21 CFR 211 sets minimum GMP requirements for drug product manufacturing and testing today.
Together, they guide compliant operations, audit readiness, and reliable batch release across global supply chains.
EU GMP adds detailed guidance for quality oversight, change control, deviations, and CAPA.
21 CFR 211 focuses on written procedures, lab controls, recordkeeping, and investigations.
EU GMP — Key Production Requirements
EU GMP sets strict controls for facilities, processes, and documentation. Teams follow approved procedures and keep records complete. They prevent contamination, mix-ups, and cross-contamination.
Control clean areas, flows, and hygiene.
Use qualified equipment and calibrated instruments.
Apply change control and investigate deviations.
Maintain complete batch records and QA review.
FDA 21 CFR 211 — Key Production Requirements
FDA 21 CFR 211 requires controlled operations for drug product manufacturing. Teams use written procedures, trained staff, and reliable equipment. They document actions and investigate failures quickly.
Maintain master production records and batch production records.
Control components, containers, labeling, and storage conditions.
Run in-process controls, lab tests, and documented investigations.
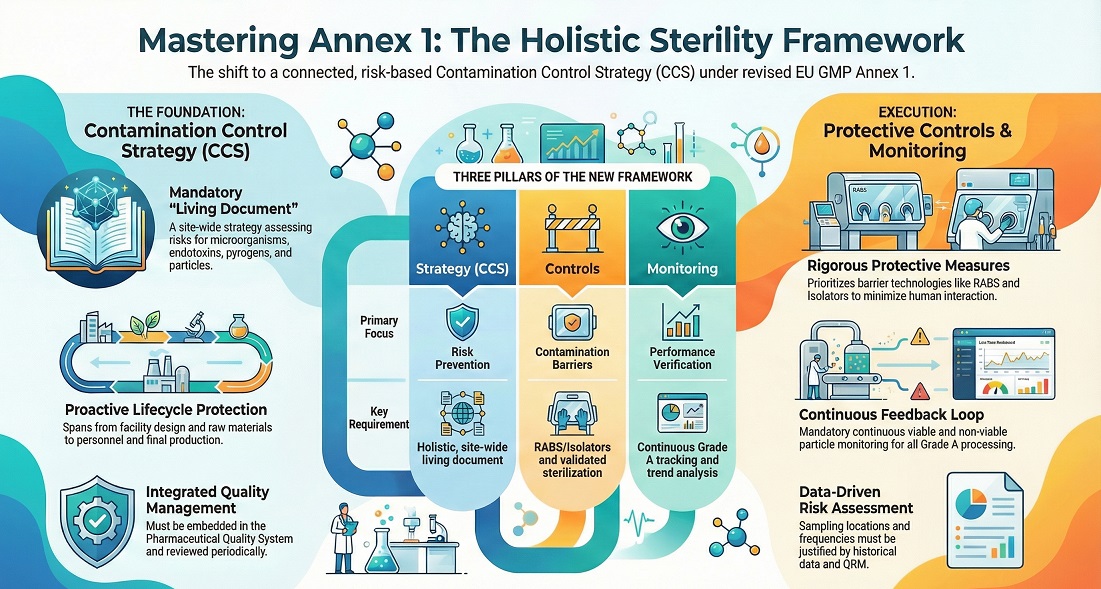
Sterile and Aseptic Manufacturing — EU GMP Annex 1, CCS, Cleanroom Grades A–D
Sterile manufacturing protects products from microbes and particles. Aseptic processing keeps sterile materials sterile during filling. EU GMP Annex 1 expects a Contamination Control Strategy (CCS). Teams map risks, define controls, and verify performance. Cleanroom grades A–D set environmental limits by activity. Teams monitor pressure and particles.
Grade A supports critical aseptic zones; Grade B supports surrounding background.
Grade C and Grade D support less critical steps and staging.
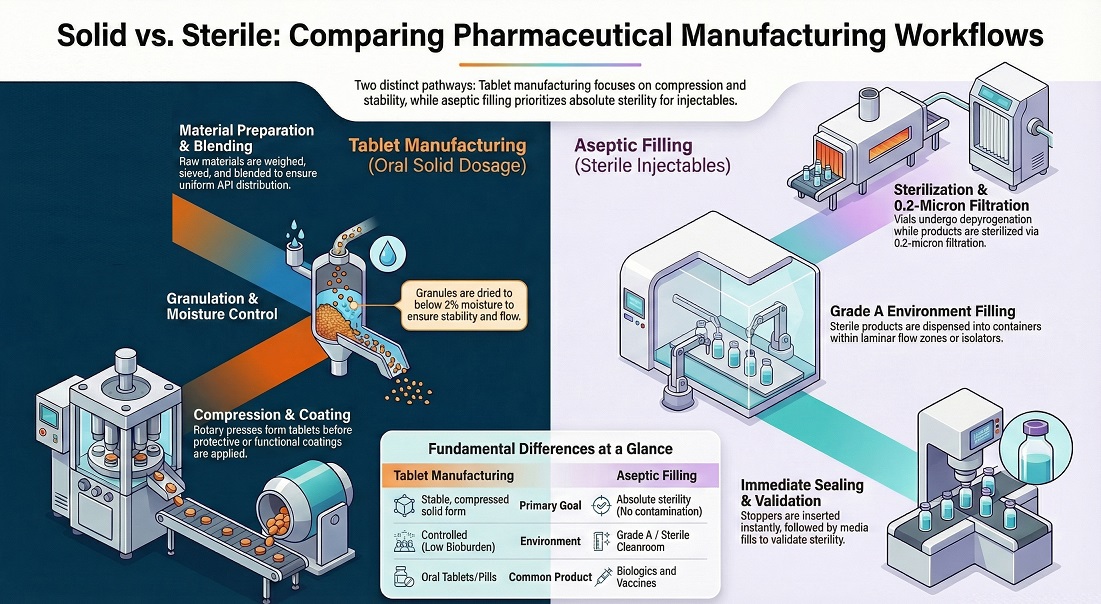
Pharma Manufacturing Process Steps — Tablet Manufacturing and Aseptic Filling Workflows
Tablet workflows turn powders into stable oral doses. Aseptic workflows fill sterile product without contamination. Teams follow validated steps and record key parameters.
Weigh and dispense materials, then mix and blend to meet uniformity.
Granulate or direct-compress, then dry and mill to control flow.
Compress into tablets, then coat and print to protect and identify.
Sterile filter or prepare solution, then fill, stopper, cap, and inspect vials.
Final Words
Pharma industry turns validated processes into consistent, compliant batches under a quality system. ICH Q10 (2008) defines the Pharmaceutical Quality System model; ICH Q9(R1) (2022) guides risk decisions across the lifecycle. WHO estimates 1 in 10 medicines in LMICs are substandard or falsified, costing US$30.5B each year.
Use the Pharma Production Planner Career Path to map roles, requirements, and process steps. EU GMP guidance lives in EudraLex Volume 4. In sterile operations, Annex 1 makes one point clear: the Contamination Control Strategy must cover the facility, linking design, procedures, and monitoring into one evidence set.
FAQs
A planner links the schedule to GMP controls: approved materials, trained staff, qualified equipment, and complete batch records. They plan around line clearance, cleaning, and QA release to avoid delays.
A CCS is the site’s integrated contamination plan. It defines critical control points and checks the effectiveness of design, procedures, and monitoring—across the facility.
The European Commission publishes EU GMP guidance in EudraLex Volume 4, including Part I chapters (e.g., Documentation, Production, Quality Control) and GMP-related documents.
References

Ershad Moradi
Ershad Moradi, a Content Marketing Specialist at Zamann Pharma Support, brings 6 years of experience in the pharmaceutical industry. Specializing in pharmaceutical and medical technologies, Ershad is currently focused on expanding his knowledge in marketing and improving communication in the field. Outside of work, Ershad enjoys reading and attending industry related networks to stay up-to-date on the latest advancements. With a passion for continuous learning and growth, Ershad is always looking for new opportunities to enhance his skills and contribute to pharmaceutical industry. Connect with Ershad on Facebook for more information.

Master GxP Validation in 2026: Meaning, Key Steps, and Validated State Control
Auditors want evidence you can trace, not opinions you can explain. GxP validation links intended use, requirements, risk, and test results into one story. When you control changes and review performance, you keep the system inspection-ready every day on time.

Master GMP Compliance in 2026: Meaning, Core Elements, and How to Implement
GMP compliance keeps medicines safe, consistent, and traceable across every batch. This guide explains core GMP elements, practical rollout steps, and common pitfalls. It also shows how to strengthen training, documentation, data integrity, and audit readiness.

History of Pharmacovigilance: From the Thalidomide Crisis (1961–2026) to GMP Oversight
Thalidomide in 1961 changed drug safety forever. Since then, pharmacovigilance has grown from crisis response to proactive risk management. Today, teams track signals, tighten reporting rules, and connect safety data to quality systems. As a result, PV now links directly to GMP oversight, audits, and data integrity.


