Curious about what is GMP certified and why everyone in regulated industries talks about it? You’re not alone. Whether you’re launching a supplement brand, managing a pharmaceutical facility, or aiming to boost your career, understanding GMP (Good Manufacturing Practice) certification is crucial.
This in-depth guide covers every angle. You’ll learn what GMP certification means, how the FDA enforces it, what steps you need to take to earn it, and why it benefits your organization or personal brand. We’ll also explore ISO and global standards, renewal cycles, and practical ways to stay compliant.
By the end, you’ll not only know the theory—you’ll have actionable tips to help you achieve, maintain, and leverage GMP certification for success in 2025 and beyond.

What Is GMP Certified?
Being GMP certified proves that a facility, product, or process follows strict Good Manufacturing Practice standards. These standards keep products safe, consistent, and high-quality in every batch. They also protect consumers and build trust with partners and regulators.
The FDA enforces cGMP rules for pharmaceuticals and medical devices to ensure safety and effectiveness. For supplements, foods, and cosmetics, businesses often work with third-party audits like NSF/ANSI 455 to achieve certification.
Related Resources on Pharmuni:
Sign up for Introduction to Good Manufacturing Practices (GMP) FREE Course
The GMP Certified Process and Renewal
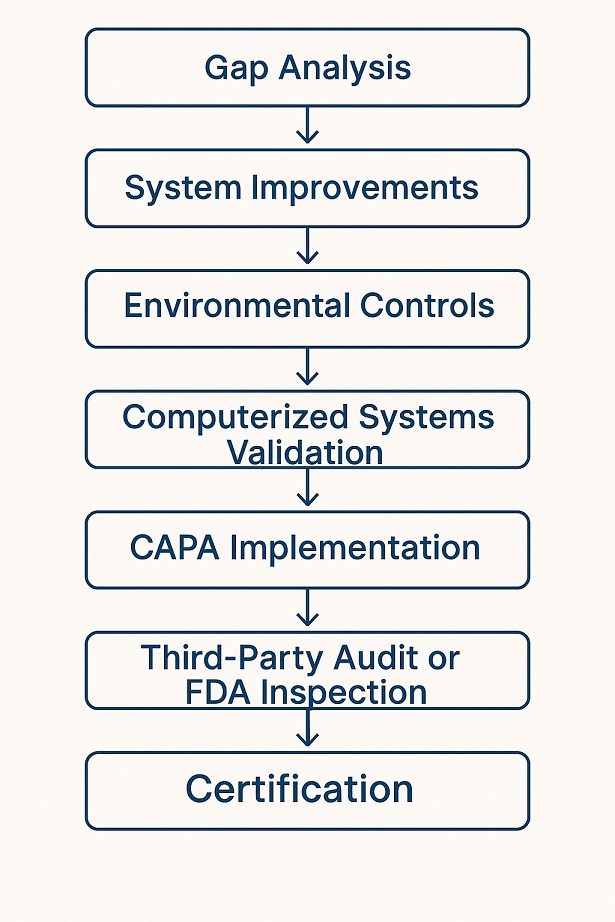
Certification Process Steps
Earning GMP certified status starts with a clear gap analysis. First, evaluate your operations against all GMP requirements. Then, identify weaknesses and prioritize them for improvement. After that, update your SOPs, validate all processes, and train employees thoroughly. Each improvement builds a stronger quality system and prepares your team for success. Moreover, review every department to ensure consistent compliance across the organization.
Next, focus on environmental controls to maintain cleanrooms, HVAC systems, and monitoring tools. Then, validate computerized systems (CSV) to ensure software supports data integrity and compliance. Afterward, implement CAPA programs to address deviations quickly and effectively. Furthermore, prepare for a third-party audit or an FDA inspection with organized records. This step proves your systems meet high standards.
Finally, after successful review, you earn official documentation that confirms you are GMP certified.
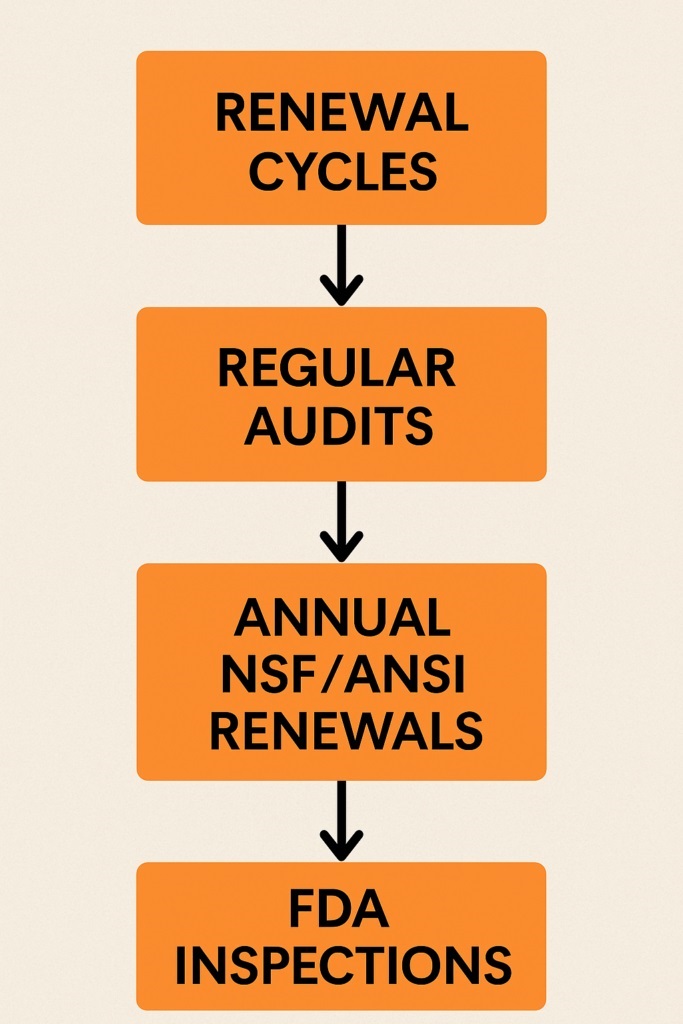
Renewal Cycles
Staying GMP certified requires focus and consistent effort. First, schedule regular audits to check compliance in every department. Then, review processes and documentation to find gaps or risks early.
Moreover, train employees frequently to keep everyone updated on current GMP standards. After that, address issues quickly to prevent larger problems during inspections.
In addition, track performance metrics to measure improvements and build a stronger quality culture.
Next, plan for annual renewals if you hold NSF/ANSI certifications. Review all requirements and prepare updated records before the audit. Furthermore, understand that FDA compliance is ongoing, not a one-time process.
Therefore, expect periodic inspections, especially after major facility or process changes. Always document updates and maintain accurate records to prove compliance.
Finally, encourage continuous improvement initiatives to strengthen systems and reduce risks.
Benefits of Being GMP Certified
Builds trust and credibility
Customers, regulators, and partners recognize your commitment to safety and quality.
Ensures product consistency
Every batch meets the same strict standards for quality and safety.
Unlocks new market opportunities
Many global markets and retailers only accept GMP certified products.
Streamlines audits
Organized processes and records make regulatory inspections faster and easier.
Improves operational efficiency
Validated systems and trained teams reduce errors and downtime.
Standards and Global Context
ISO 13485 vs GMP for Medical Devices
Medical device makers rely on GMP certified systems and ISO 13485 to ensure quality. GMP focuses on production consistency, traceability, and employee training for every batch.
Meanwhile, ISO 13485 strengthens design, development, and risk management processes.
Together, these standards improve safety, reduce risks, and support global market access, giving manufacturers a strong foundation for compliance and customer trust.
Leverage free GMP education
WHO and EU GMP share the same core principles as FDA cGMP but include region-specific nuances.
Aligning with these standards simplifies global trade and ensures compliance across multiple jurisdictions.
Common Audit Findings to be GMP Certified
Supplier Qualification Gaps – Missing or incomplete vendor audits.
Training Records – Outdated or incomplete personnel training logs.
Documentation Issues – Inconsistent or missing records.
Environmental Monitoring – Gaps in data or equipment calibration.
CAPA Inefficiencies – Weak root cause analysis or poor follow-up.
Building a Quality Culture in GMP Environmet
Understanding what is GMP certified is just the start. Sustaining compliance means embedding quality into your organization.
People
Train and retrain employees regularly.
Processes
Document and validate everything.
Procedures
Update SOPs as regulations evolve.
Plant
Maintain clean and controlled environments.
Practices
Perform regular internal audits and continuous improvement reviews.
GMP Training and Competency Development
Training is central to certification and compliance. Programs cover:
- SOP Adherence – Everyone follows standardized processes.
- CAPA – Employees understand how to manage and document deviations.
- CSV – IT teams validate systems to ensure data integrity.
- Audit Preparation – Teams practice for inspections to reduce anxiety and errors.
For career growth, consider enrolling in GMP e-learning or attending ISPE conferences to stay ahead.
Colclusion
What is GMP certified? It’s more than a label. It’s a commitment to safety, quality, and trust.
For professionals, it’s a chance to enhance your resume and build authority. For businesses, it ensures compliance, market access, and consumer confidence.
By staying informed, investing in training, and embedding quality into your culture, you’ll position yourself—and your organization—for sustainable success in regulated industries.
Take the next step today. Explore Pharmuni’s Career Path Guide and unlock opportunities to grow your expertise and your business.

Ershad Moradi
Ershad Moradi, a Content Marketing Specialist at Zamann Pharma Support, brings 6 years of experience in the pharmaceutical industry. Specializing in pharmaceutical and medical technologies, Ershad is currently focused on expanding his knowledge in marketing and improving communication in the field. Outside of work, Ershad enjoys reading and attending industry related networks to stay up-to-date on the latest advancements. With a passion for continuous learning and growth, Ershad is always looking for new opportunities to enhance his skills and contribute to pharmaceutical industry. Connect with Ershad on Facebook for more information.

Master GxP Validation in 2026: Meaning, Key Steps, and Validated State Control
Auditors want evidence you can trace, not opinions you can explain. GxP validation links intended use, requirements, risk, and test results into one story. When you control changes and review performance, you keep the system inspection-ready every day on time.

Master GMP Compliance in 2026: Meaning, Core Elements, and How to Implement
GMP compliance keeps medicines safe, consistent, and traceable across every batch. This guide explains core GMP elements, practical rollout steps, and common pitfalls. It also shows how to strengthen training, documentation, data integrity, and audit readiness.

History of Pharmacovigilance: From the Thalidomide Crisis (1961–2026) to GMP Oversight
Thalidomide in 1961 changed drug safety forever. Since then, pharmacovigilance has grown from crisis response to proactive risk management. Today, teams track signals, tighten reporting rules, and connect safety data to quality systems. As a result, PV now links directly to GMP oversight, audits, and data integrity.




