Pharma companies operate under strict regulations. They must follow Good Manufacturing Practice (GMP), GxP, and international quality standards. A powerful compliance management system (CMS) helps ensure that every step of production meets these regulations. It’s not optional—it’s a must for staying competitive, audit-ready, and legally safe. In highly regulated environments, a compliance management systems bring structure, visibility, and accountability.
It centralizes documentation, tracks processes, and automates reporting. Pharma companies that lack such systems risk costly deviations, product recalls, and even regulatory sanctions. If you want to stay audit-ready and efficient, now is the time to adopt or optimize your compliance system.

What Is the Role of a Compliance Management System in Pharma?
In pharma, every product must meet strict industry and legal standards—and a CMS makes that possible. It connects quality processes with real-time data tracking and audit readiness.
Companies use it to:
- Maintain GMP standards.
- Meet GxP & Validation requirements.
- Align with frameworks like Computerized System Validation (CSV) and Title 21 CFR Part 11 Compliance.
- Track deviations, corrective actions, and supplier performance.
Modern systems integrate Good Automated Manufacturing Practice (GAMP) principles and validate IT systems in labs. Whether you’re managing clinical trials or manufacturing units, compliance systems are vital.
Sign up for Planning Phase for Production Batch Management Course
Key Features in a Pharma Compliance Management System
Electronic Trial Master File (eTMF)
Store essential clinical documentation.
Audit Trails
Capture every change for regulatory proof.
Deviation Management
Flag and resolve process inconsistencies.
CAPA Workflows
Automate root cause analysis and prevention.
Training Management Modules
Track employee readiness and certification.
How Does a Compliance System Improve Pharma Operations?
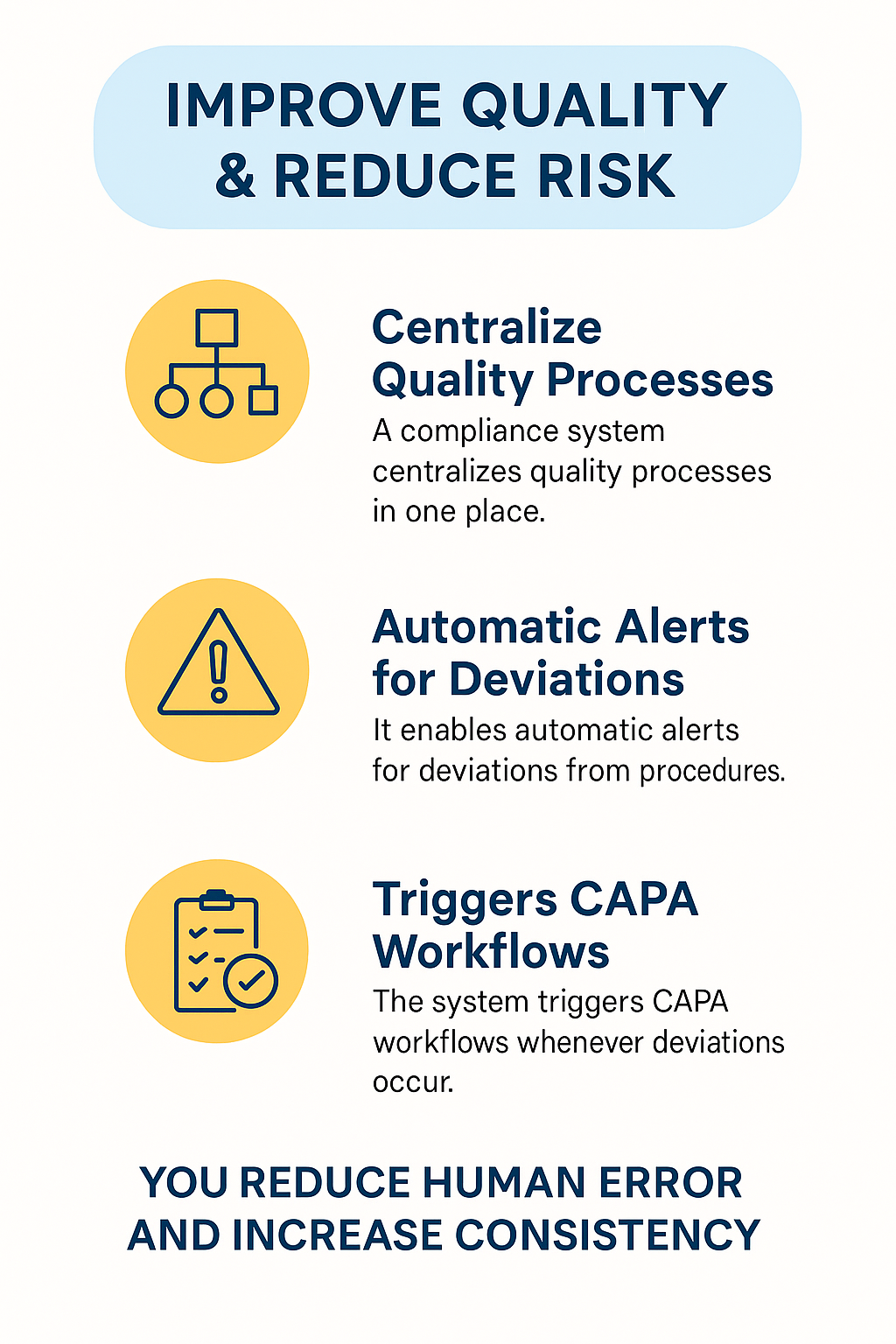
Improve Quality & Reduce Risk
A CMS helps pharma companies improve quality and reduce risk quickly. It connects all quality processes into one platform, making it easier to manage daily operations.
As a result, you can spot errors early and take corrective action before they grow. The system alerts your team when something goes wrong, so you respond right away. In every step, it keeps records, tracks changes, and ensures everyone follows the right procedures.
Moreover, the system reduces manual work and eliminates confusion. You don’t waste time searching for files or double-checking spreadsheets. Instead, you focus on real issues and solve them faster.
CAPA workflows launch automatically when a deviation occurs, helping your team act with precision. Over time, you build a stronger quality culture across all departments.
This consistent approach keeps you audit-ready and ensures long-term compliance. With better control and fewer risks, your pharma operations become more reliable and efficient.
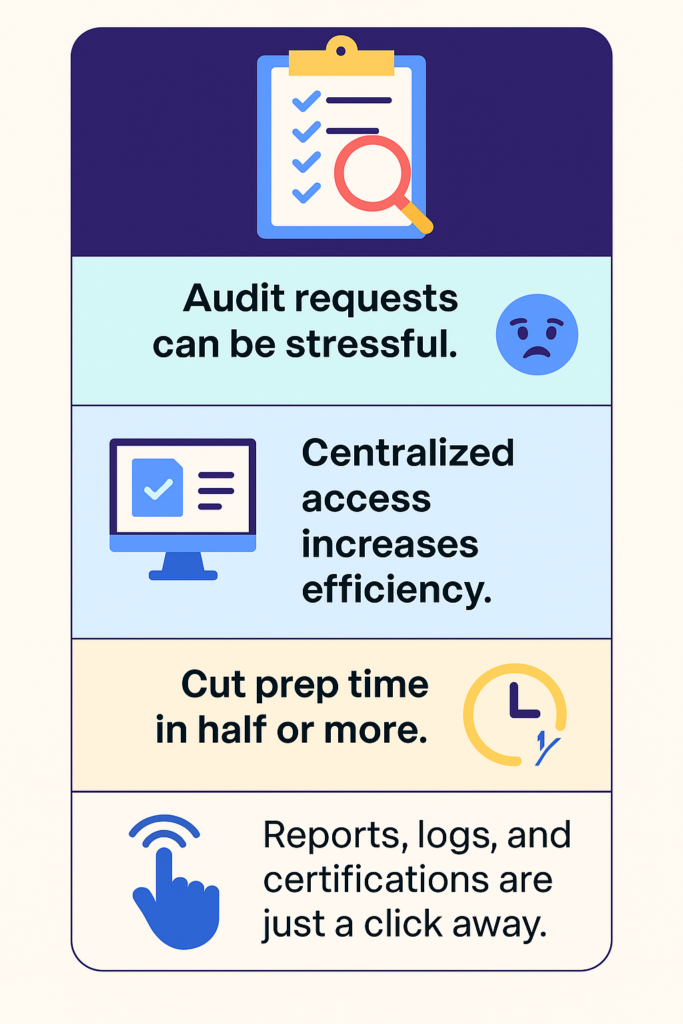
Speed Up Audit Readiness
Audit requests often create pressure and disrupt daily operations. However, a compliance management system solves this problem fast.
It stores all reports, logs, and training records in one secure location. So, when an audit begins, you don’t waste time searching. Instead, you access everything with a few clicks. This instantly cuts preparation time by 50% or more.
You feel confident because every document stays current and easy to retrieve. Moreover, the system updates records in real time, so nothing gets lost or delayed.
In addition, the system improves how your team handles inspections. It displays audit trails, completed CAPAs, and training statuses in seconds. As a result, you respond to auditor questions clearly and quickly.
Every file includes the correct version and timestamp, which builds trust.
Over time, this improves your audit outcomes and reduces non-compliance risks. Therefore, you save time, protect your license, and boost team performance. With the right system, audits become routine—not a surprise.
What Are the 6 Compliance Goals Pharma Must Achieve?
Meet GMP and GxP Requirements
Follow Good Manufacturing Practice and quality frameworks.
Enable Real-Time Traceability
Track materials, batches, and changes.
Automate Documentation
Reduce paper records and improve access.
Strengthen Quality Culture
Promote training and accountability.
Ensure Data Integrity
Validate data inputs and enforce user controls.
How Do Compliance Management Systems Handle Emerging Challenges?
Cybersecurity & Data Access Control
Pharma companies handle sensitive data every day, including clinical, batch, and employee information. Therefore, they must protect it at all times. A strong compliance management system provides built-in cybersecurity features that guard against threats. It uses encryption to secure files and prevent data leaks. In addition, role-based access control ensures only the right people see specific information. So, unauthorized users can’t enter or change critical documents. Backups run automatically, so you never lose important data. These tools give your team confidence and keep regulators satisfied.
Moreover, cybersecurity in pharma is not optional anymore—it’s essential. Regulators now expect companies to follow strict digital protection standards. Without proper controls, one breach can lead to serious legal issues. A modern compliance system keeps your data safe, even during audits or system updates. It also logs every action, so you can trace who did what and when. This improves accountability and protects against fraud. With secure access and full control, your company stays audit-ready and trustworthy.

Automation & AI for Compliance
Automation and AI change how pharma teams handle compliance every day. Instead of checking data manually, teams now use AI tools to speed up their work. These tools scan huge amounts of data in seconds and detect errors or trends early. As a result, your team fixes problems faster and avoids delays. AI also sends real-time alerts when something looks unusual. So, you don’t miss key issues or fall behind on deadlines. In every process, automation adds speed, accuracy, and control.
Moreover, predictive analytics improves how you plan future batches. The system analyzes past data to find patterns and forecast risks. This helps you avoid common production issues before they happen. Teams use this insight to improve efficiency and reduce waste. You can also automate routine tasks like generating audit reports or tracking CAPAs. With less manual work, your team focuses more on quality. Over time, automation helps your company stay compliant, save time, and respond faster to regulatory changes.
How Does a Compliance Management System Compare to Manual Methods?
Real-Time Dashboards vs. Spreadsheets
Manual spreadsheets get outdated fast. Compliance dashboards refresh in real time and support instant data review.
Centralized Access vs. Siloed Tools
With CMS, all SOPs, audit logs, and training records stay in one place. No need to chase files across departments.
What Compliance Features Should You Prioritize First?
CAPA Workflow Systems
Reduce repeat deviations with consistent root cause investigations.
Deviation Management Tools
Log and manage all unexpected events.
Quality Metrics Dashboards
Track performance trends and detect anomalies.
Real-Time Alerts
Receive notifications when compliance is at risk.
Training Integrations
Connect learning modules directly to roles and systems.
Conclusion
Based on this article , a compliance management system isn’t just a tech upgrade—it’s a pharma business necessity. You’ll boost operational efficiency, reduce audit stress, and strengthen regulatory trust.
Whether you’re in QA, batch management, or regulatory affairs, learning how compliance works is crucial. Don’t wait for an audit to show you the gaps.
Take the first step

Ershad Moradi
Ershad Moradi, a Content Marketing Specialist at Zamann Pharma Support, brings 6 years of experience in the pharmaceutical industry. Specializing in pharmaceutical and medical technologies, Ershad is currently focused on expanding his knowledge in marketing and improving communication in the field. Outside of work, Ershad enjoys reading and attending industry related networks to stay up-to-date on the latest advancements. With a passion for continuous learning and growth, Ershad is always looking for new opportunities to enhance his skills and contribute to pharmaceutical industry. Connect with Ershad on Facebook for more information.

Effortlessly Boost Compliance with CSV Pharm Tech
Discover how CSV pharm tech helps pharma professionals manage validation, compliance, and data integrity with confidence. Learn how Pharmuni’s CSV course can help you grow your pharma career.

Top 5 Non-Boring Annex 1 Training Ideas for New Hires
Annex 1 training doesn’t have to be boring. Learn five creative ways to teach sterile manufacturing rules that new hires will remember. From storytelling to interactive quizzes, these strategies turn compliance into an engaging, practical learning experience.

Best GMP Certified Supplements to Buy in 2025
GMP certified supplements guarantee safety and consistency. Learn how to identify, compare, and buy the best ones, including expert tips and trusted resources from Pharmuni.



