Audit gaps rarely start with a big mistake. Instead, they start with small “system” issues like unclear SOP ownership, weak document control, or messy training evidence. However, auditors do not treat them as small. In fact, FDA data show 621 foreign and 444 domestic drug manufacturer inspections in FY2023—that is 1,065 inspection opportunities for a gap to surface. Therefore, quality teams need a practical way to align expectations across departments, suppliers, and sites. That is exactly why this GMP vs ISO comparison matters.
Quality also carries real-world consequences beyond audits and certificates. For example, WHO estimated that 1 in 10 medicines in low- and middle-income countries fail quality tests, which points to substandard or falsified products. So, pharma teams must control processes, records, and risks with discipline. Meanwhile, GMP and ISO often confuse teams because both talk about QMS, yet each drives different behaviors. In this article, you will learn where GMP sets out strict regulatory control and where ISO 9001 strengthens system performance and improvement. Start here for GMP fundamentals: Good Manufacturing Practices (GMP)
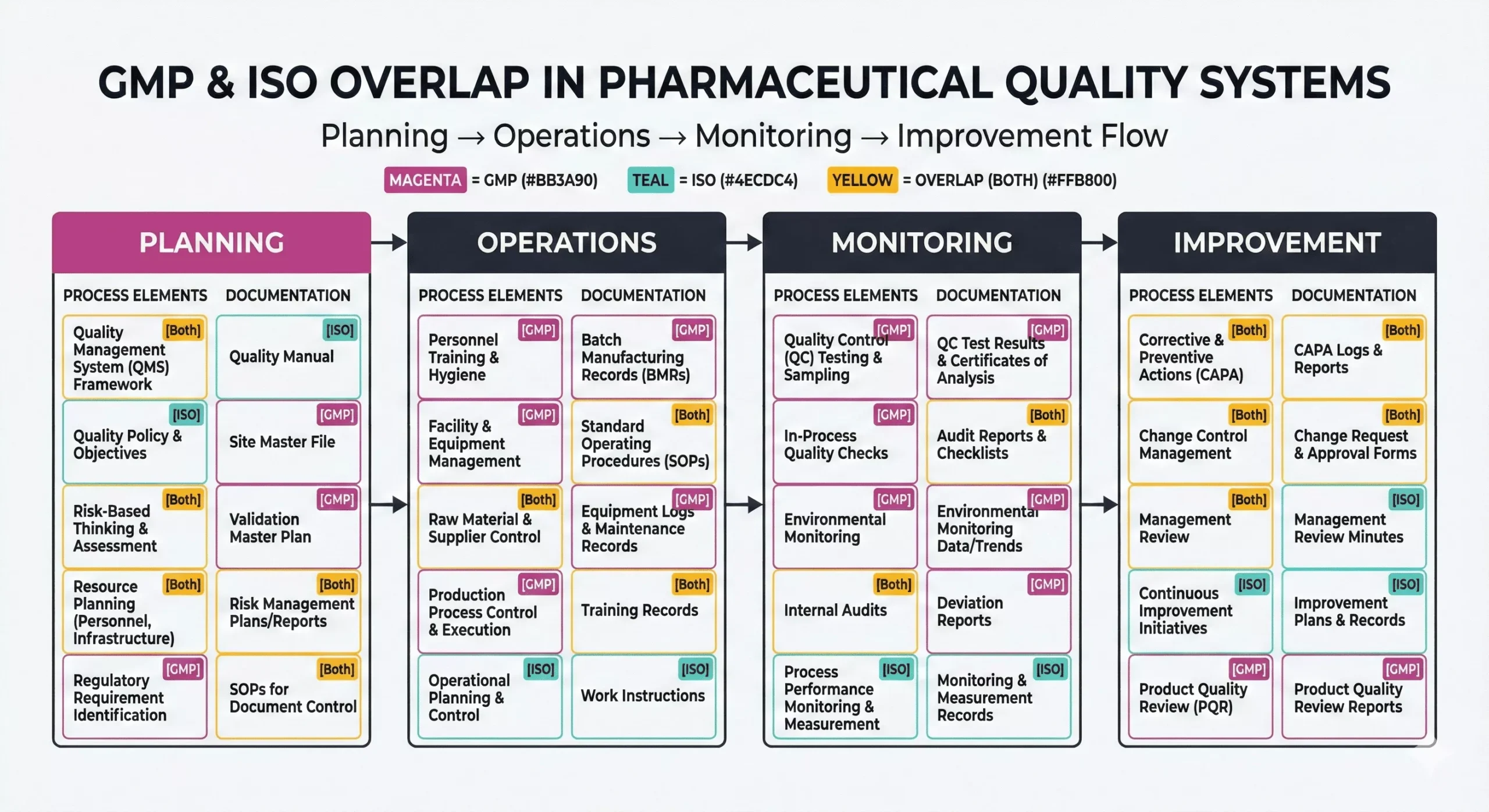
Key Differences Between GMP vs ISO in Pharma Quality Systems
GMP and ISO both support quality management, yet they serve different purposes. GMP sets out regulatory expectations for how you make, test, store, and distribute medicines. ISO 9001 sets out a general QMS model that helps any industry improve consistency and customer satisfaction. Therefore, GMP focuses on product control and compliance, while ISO focuses on system control and continual improvement.
Think of GMP and ISO like this:
- GMP/ISO standards: GMP protects the patient; ISO 9001 strengthens the QMS.
- GMP Compliance and ISO Certification: GMP follows laws and inspections; ISO follows third-party certification audits.
- Pharmaceutical Quality Systems: strong sites combine both, then reduce variation and deviations.

How GMP vs ISO Define and Control Pharmaceutical Quality
GMP links “quality” to meeting product specifications and controlling variability. So teams prove control through validated processes, controlled materials, trained people, and reliable data. ISO 9001 links “quality” to meeting requirements consistently and improving performance. In addition, ISO pushes a “process approach,” so teams map inputs, outputs, owners, and metrics.
As a result, GMP and ISO use different quality lenses:
- GMP prioritizes patient and product risk
- ISO prioritizes process effectiveness and customer needs.
Documentation and Process Requirements Under GMP vs ISO
Both frameworks demand documentation control, but GMP demands deeper traceability. GMP requires detailed, contemporaneous records that show what happened in each batch step. ISO 9001 requires “documented information” that supports process control, audits, and improvement.
Use this checklist to compare GMP and ISO documentation:
GMP typically expects:
- Batch records and reconciliation, plus traceability to materials and equipment.
- Deviations, investigations, and CAPA with strong root-cause evidence.
- Change control with impact assessment and validation decisions.
- Qualification, calibration, and validation evidence for critical equipment and systems.
ISO 9001 pharma programs typically expect:
- A clear process map, roles, objectives, and performance evidence.
- Document control, internal audits, management review, and corrective actions.
- Supplier evaluation and monitoring that matches risk.
Risk Management Approaches
Risk thinking appears in both, yet GMP uses formal quality risk management more often. For example, teams run risk assessments for validation scope, cleaning limits, supplier qualification, and change control. ISO 9001 also requires risk-based thinking, but it gives flexibility on tools and depth.
Many pharma teams align GMP risk work with ICH Q9 quality risk management, which regulators reference in guidance. Consequently, GMP and ISO risk work often looks like this:
- GMP emphasizes product and data risk, then documents rationales for critical decisions.
- ISO emphasizes process and business risk, then plans actions and measures outcomes.
Operational Impacts of GMP vs ISO on Daily Pharma Activities
On the shop floor, GMP drives “must-do” routines: line clearance, label checks, status labeling, segregation, and real-time recording. Meanwhile, teams confirm calibration status before they measure or release. Also, sites control environments and sampling where product risk demands it.
ISO 9001 shapes daily work through targets, reviews, and improvement routines. Therefore, supervisors track KPIs, investigate performance drift, and remove recurring causes. In addition, leaders use trend reviews to prioritize resources.
You can spot GMP/ISO in one simple contrast:
- GMP asks for evidence per batch.
- ISO asks for evidence the system works overtime.
Audit and Compliance Expectations in GMP vs ISO Systems
GMP audits come from regulators and customers, and they can affect your license to supply product. FDA describes CGMP as minimum requirements for methods, facilities, and controls for drug manufacturing. In the EU, EudraLex Volume 4 sets GMP expectations and annexes for manufacturing sites.
ISO audits come from certification bodies, and they focus on QMS conformance and effectiveness. However, ISO certification never replaces GMP compliance. Instead, ISO certification can make audits smoother when teams run strong routines.
So GMP and ISO differ in consequences:
- GMP failures can delay release, trigger recalls, or escalate enforcement.
- ISO failures can suspend a certificate and damage customer trust.
When to Use GMP vs ISO in Pharma and How They Complement Each Other
Use GMP whenever you make, test, package, or distribute regulated medicines. Instead, add ISO 9001 when you want a scalable QMS that strengthens consistency across departments. Consequently, many companies integrate both: they keep GMP controls tight for regulated work, and they use ISO routines to improve planning, review, and improvement.
ISO often adds the most value in “support” processes that still affect GMP outcomes. For example, you can use ISO to strengthen:
- Supplier qualification workflows and performance scorecards,
- Onboarding, training, and competency management,
- IT and data governance that supports documentation control,
- Complaint handling and customer feedback loops.
A practical integration approach connects document control, training, supplier management, deviation/CAPA, and management review into one QMS. In addition, ISO 9001 remains the 2015 edition with a climate-action amendment in 2024, which adds context-related expectations that can influence risk discussions.
Use this quick playbook to align GMP and ISO:
- Run one document system: One change control, one training matrix, one archive rule.
- Define “critical” clearly: Tighten controls for GMP-critical data, equipment, and steps.
- Upgrade CAPA discipline: Trend repeat issues and verify effectiveness every time.
- Audit smart: Map internal audits to both frameworks and rank findings by risk.
Final words
In pharma, quality gaps rarely stay small. However, regulators spot them fast when records drift or controls weaken. For example, FDA data show 621 foreign and 444 domestic drug manufacturer inspections in FY2023 (1,065 total). Meanwhile, WHO estimates 1 in 10 medicines in low- and middle-income countries are substandard or falsified. Therefore, you should treat GMP and ISO as a practical alignment topic, not a debate.
ISO also matters because teams use it at massive scale. In fact, the ISO Survey reported 1,265,216 ISO 9001:2015 certificates worldwide in 2022. So you can use ISO to strengthen your QMS rhythm—KPIs, management review, internal audits—while you keep GMP controls strict for batch quality and patient safety. Also set a clear target, such as 40% fewer repeat deviations, then track it monthly.
FAQ:
Yes. Regulators enforce GMP for medicines, while organizations choose ISO certification.
No. ISO supports a QMS, but regulators still expect full GMP controls.
GMP usually requires deeper, batch-level records and stronger data traceability.
References:

Stephanie Männicke
Digital Marketing Especialist at Zamann Pharma Support, brings 8 years of experience in Corporate and Digital Communication. Specializing in Digital Marketing and Content Creation, Stephanie is currently focused on creating strategic content for Pharmuni's networks, especially content on topics such as recruitment, onboarding and employer branding. Outside of work, Stephanie is a mum, a crocheter and a movie fan. An avid reader and in search of expanding her knowledge, Stephanie is always looking for ways to innovate communication in the digital environment and connect people in a genuine way.

Master GxP Validation in 2026: Meaning, Key Steps, and Validated State Control
Auditors want evidence you can trace, not opinions you can explain. GxP validation links intended use, requirements, risk, and test results into one story. When

Master GMP Compliance in 2026: Meaning, Core Elements, and How to Implement
GMP compliance keeps medicines safe, consistent, and traceable across every batch. This guide explains core GMP elements, practical rollout steps, and common pitfalls. It also

History of Pharmacovigilance: From the Thalidomide Crisis (1961–2026) to GMP Oversight
Thalidomide in 1961 changed drug safety forever. Since then, pharmacovigilance has grown from crisis response to proactive risk management. Today, teams track signals, tighten reporting


