Are you ready to transform your quality management processes overnight? If so, this post is for you. In today’s fast-paced pharmaceutical and manufacturing sectors, staying ahead means leveraging the best tools available. In particular, QMS Software solutions can revolutionize how you handle Electronic Quality Management System (eQMS) tasks, from Cloud-based QMS software deployment to Access controls and permissions in QMS. You’ll also discover how Audit management software, Change management in QMS, and Compliance management systems integrate seamlessly.
Looking for a deep dive into validation? Don’t miss our Introduction to Computer Systems Validation (CSV) Course—designed for compliance professionals who want to master 21 CFR Part 11 compliance and FDA regulations integration.
Ready to boost efficiency? Enroll in the CSV course today.
What Is QMS Software?
QMS Software isn’t just another acronym. Rather, it’s a powerful suite of tools—like Document control systems, Corrective and Preventive Actions (CAPA) software, and Audit trail features—that streamline quality processes and ensure compliance. Today, digital transformation demands agile solutions: Electronic signature in QMS, Real-time quality monitoring, and KPI tracking in QMS are no longer optional. Instead, they’re essential to meet stringent regulations such as ISO 13485 QMS software standards.
Modern businesses, especially in pharmaceuticals, rely on Supplier management software, Training management in QMS, and Incident management systems to prevent costly errors.
By integrating Design control software, Design history file management, and Quality analytics dashboards, companies can optimize product launches, minimize risk, and maintain Regulatory compliance QMS seamlessly.
💡 Info: Did you know that companies using Cloud-based QMS software report a 30% reduction in audit preparation time?
Sign up for Introduction to Computer Systems Validation Course
Main QMS Software Components Should You Know?
Document Control Systems
Ensure every document—from SOPs to batch records—is current, approved, and archived properly.
CAPA Software
Automate identification and investigation of nonconformances, driving continuous improvement.
Audit Management Software
Schedule, conduct, and track internal and external audits, ensuring Audit readiness and scheduling.
Training Management in QMS
Track employee competencies, assign courses, and maintain records for 21 CFR Part 11 compliance.
Supplier Quality Management Systems
Monitor supplier performance, onboarding, and integration to prevent supply chain disruptions.
Where Does Implementation Begin?
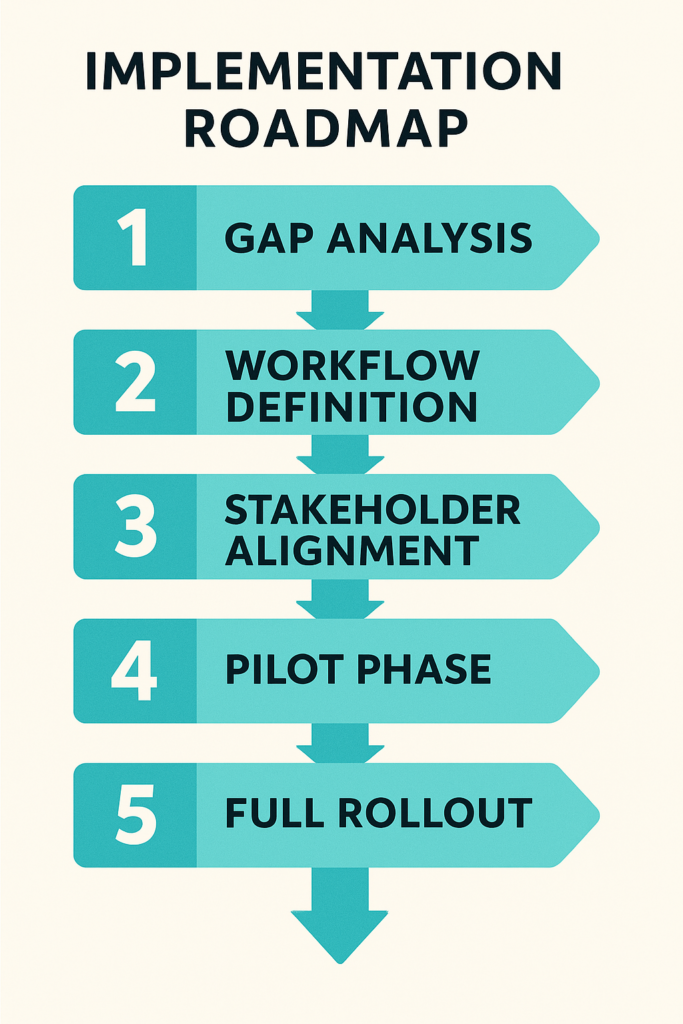
QMS Software Implementation Strategy
Building a robust QMS Software environment starts with a clear roadmap.
First, conduct a gap analysis to compare current practices against regulatory standards such as ISO 9001 requirements software and FAR (Federal Acquisition Regulation) compliance.
Next, define workflows for Document control systems, CAPA effectiveness metrics, and Incident management systems. Engage cross-functional teams—quality, IT, operations—to gather requirements on Change management in QMS, Traceability management in QMS, and Permission-based access in QMS. This ensures everyone’s on board from day one.
By focusing on Supplier onboarding processes, Supplier quality management systems, and Customer complaint management, you reduce rework and expedite approvals. Meanwhile, embedding Risk assessment integration and Quality analytics dashboards creates a proactive culture, enabling continuous monitoring.
Leveraging features like Electronic signature in QMS and Audit trail features ensures 21 CFR Part 11 compliance without manual headaches.
Implementing a Robust eQMS vs. Traditional QMS
Choosing between on-premise and Cloud-based QMS software significantly impacts implementation. On-premise solutions often demand heavy upfront investment in hardware, IT support, and Design control software licensing.
In contrast, Cloud-based QMS software provides scalability, automatic updates, and built-in backup—simplifying Regulatory compliance QMS.
A traditional QMS relies heavily on paper-based tracking: filing cabinets full of SOPs, manual Document version control, and time-consuming audit prep. Conversely, an Electronic Quality Management System (eQMS) centralizes SOPs, training records, and Nonconformance management in seconds.
Furthermore, modules for Corrective action planning tools and Preventive action planning tools automate workflows that once took weeks. If you want proven best practices for implementation, check out Implementing a Robust QMS in Pharma for in-depth insights.
What Are Key QMS Software Features?
QMS Software offers a comprehensive set of tools designed to improve product quality and streamline compliance processes. Below, you’ll find the most critical features that drive efficiency and ensure regulatory alignment.
Electronic Quality Management System (eQMS)
Central hub for all quality data and analytics. Moreover, eQMS enables teams to access real-time performance metrics and generate actionable reports instantly. By consolidating documents, CAPA records, and audit trails in one platform, organizations can reduce manual errors and accelerate decision-making.
Cloud-based QMS Software
Accessible anywhere, ensures real-time collaboration. Additionally, cloud deployment eliminates the need for costly on-premise infrastructure and simplifies software updates. As a result, remote teams can work together seamlessly, share documents, and maintain version control without geographic constraints.
Access Controls and Permissions in QMS
Protects sensitive data and enforces role-based access. Furthermore, granular permission settings allow administrators to assign specific read, write, or approve rights to each user. Consequently, companies can safeguard confidential information, comply with data privacy regulations, and reduce the risk of unauthorized changes.
Audit Management Software
Facilitates internal vs. external audits with ease. In addition, integrated scheduling and checklists help audit teams stay organized and complete tasks on time. This feature also generates automatic notifications and audit reports, ensuring stakeholders are informed and corrective actions are tracked promptly.
Change Management in QMS
Streamlines approvals through Change control boards QMS. Moreover, automated workflows guide each change request through defined review steps, from initiation to final approval. By maintaining a clear audit trail and version history, organizations can demonstrate regulatory compliance and prevent unauthorized modifications.
Why Choose eQMS and Not Manual Processes?
Speed and Accuracy with QMS Software
Manual processes rely on paper trails and spreadsheets, which are prone to delays and human error. For instance, tracking nonconformances on spreadsheets often leads to missed CAPA deadlines.
In contrast, QMS Software platforms automate alerts: when a nonconformance is logged, the right stakeholders are notified instantly, reducing response times by up to 50%. Built-in Audit trail features ensure every action—such as Electronic signature in QMS—is logged securely, supporting Audit readiness and scheduling. Real-time dashboards highlight KPI tracking in QMS so managers can identify trends and address issues proactively rather than post-factum.
Moreover, data integrity becomes critical during regulatory inspections. A manual system might require weeks to compile training records for 21 CFR Part 11 compliance, whereas an Electronic Quality Management System (eQMS) generates reports in minutes.
The result? Faster audit closures, fewer FDA warning letters, and improved stakeholder confidence. If you want to dive deeper into the benefits, visit our Key Elements of a Successful Pharmaceutical QMS.
Cost Savings and ROI of QMS Software
The upfront cost of Cloud-based QMS software can seem daunting, but the ROI speaks for itself. Companies report up to 40% reduction in nonconformance-related costs and a 25% improvement in on-time delivery of corrective actions. By automating Document control systems, Change management in QMS, and Supplier management software, you eliminate paper storage expenses and reduce printing by 70%.
These savings add up quickly.
Consider a mid-sized pharmaceutical firm struggling with batch release delays due to manual sign-offs.
By implementing a Pharmaceutical QMS software solution with integrated Electronic signature in QMS and Design control software, they shortened release cycles from 10 days to 3 days—freeing up valuable resources to focus on innovation. If you want to compare traditional vs. modern approaches in depth, check out QMS Quality vs Traditional Approaches: Unleashing Efficiency.
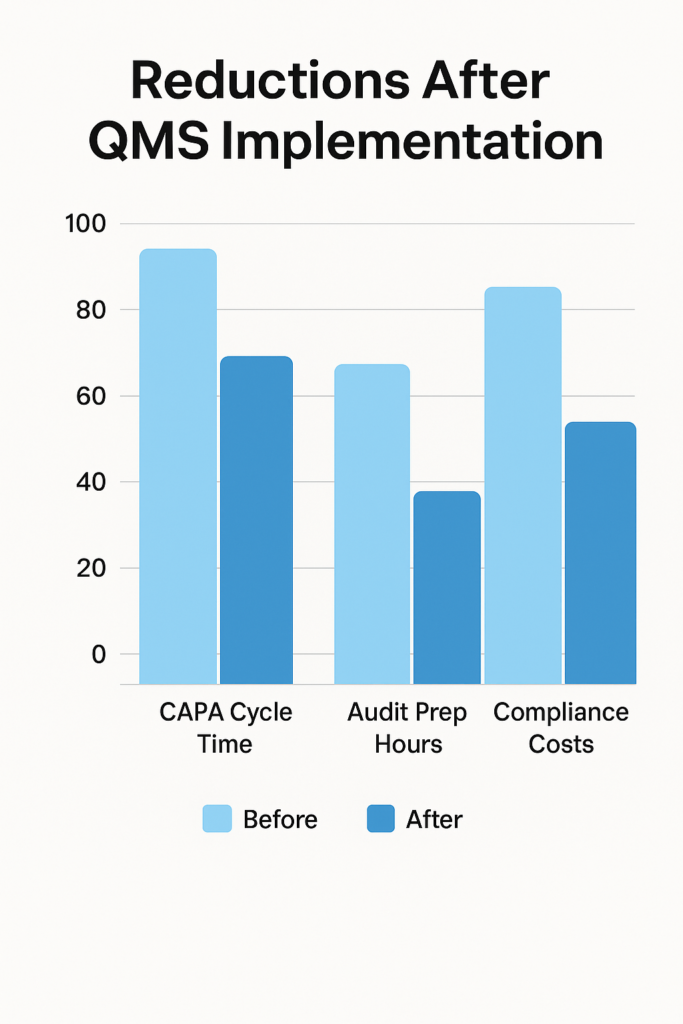
How Can You Measure QMS Software ROI?
Measuring ROI involves tracking tangible and intangible benefits. Start by comparing baseline metrics—like CAPA closure time, audit preparation hours, and compliance costs—against post-implementation figures. For example, if your organization spends 200 hours quarterly on manual Document control systems, switching to a Cloud-based QMS software can cut that workload by 75%, freeing up 150 hours for higher-value activities.
Next, quantify cost savings from improved compliance. Companies that adopt Regulatory compliance QMS and ISO 9001 requirements software often see a 20–30% drop in audit nonconformities, translating into fewer FDA warning letters and lower corrective action costs. Additionally, track revenue impacts: faster product launches due to streamlined Change management in QMS and Supplier onboarding processes accelerate time-to-market by up to 6 months, resulting in earlier sales.
Don’t forget intangible benefits: enhanced brand reputation, higher customer satisfaction through Customer complaint management, and improved employee morale as staff spend more time on value-added tasks rather than paperwork. These factors, while harder to quantify, contribute significantly to long-term growth.
Proven Best Practices for QMS Software Success
Define Clear Objectives and Scope
Establish specific goals—such as reducing CAPA cycle time by 30%—and map out which modules (e.g., Audit management software, Design control software) you need to achieve them.
Engage Stakeholders Early
Involve quality, IT, operations, and suppliers to ensure modules like Supplier quality management systems and Training management in QMS align with real-world needs.
Maintain Robust Change Control
Leverage Change control boards QMS and automated approval workflows to reduce delays and ensure all stakeholders sign off on revisions.
Prioritize Data Integrity
Implement Permission-based access in QMS and Two-factor authentication in QMS to secure sensitive data, including Quality documents management.
Conduct Regular Risk Assessments
Use Quality risk management tools to identify critical control points—especially in Medical device QMS software and Food safety QMS (HACCP) software.
Conclusion
Implementing QMS Software is no longer optional—it’s essential to remain competitive and compliant in today’s regulated industries. By adopting modern, Cloud-based QMS software solutions, you streamline Document control systems, automate Corrective and Preventive Actions (CAPA) software, and simplify Audit readiness and scheduling. You’ll also benefit from enhanced Risk assessment integration, robust Two-factor authentication in QMS, and proactive Real-time quality monitoring.
Ready to master the fundamentals? Elevate your compliance game by enrolling in our Introduction to Computer Systems Validation (CSV) Course. This interactive program covers 21 CFR Part 11 compliance, FDA regulations integration, and hands-on exercises with real-world examples. Don’t wait—discover how to validate systems effectively and stay audit-ready.
For more expert insights on Pharmaceutical QMS software, explore our blog posts:

Ershad Moradi
Ershad Moradi, a Content Marketing Specialist at Zamann Pharma Support, brings 6 years of experience in the pharmaceutical industry. Specializing in pharmaceutical and medical technologies, Ershad is currently focused on expanding his knowledge in marketing and improving communication in the field. Outside of work, Ershad enjoys reading and attending industry related networks to stay up-to-date on the latest advancements. With a passion for continuous learning and growth, Ershad is always looking for new opportunities to enhance his skills and contribute to pharmaceutical industry. Connect with Ershad on Facebook for more information.

GxP in pharma in 2026: How Inspectors Evaluate System Control
This article explains how GxP quality systems are assessed during inspections, why gaps in governance and execution lead to repeat audit findings, and how regulated pharma operations strengthen inspection readiness through risk management, data integrity, and lifecycle compliance controls.

Quality Oversight In 2026: Roles in pharma industry, GMP Expectations, And Risk-Based Control
Quality Oversight keeps GMP decisions consistent, traceable, and risk-based. It connects deviation handling, CAPA, change control, and trend reviews. It also proves management engagement through documented actions. Strong oversight reduces repeat failures and supports confident batch disposition.

Revalidation In Pharma (2026 guide): Meaning, Triggers, Frequency, And Requalification Differences
Revalidation protects product quality and business continuity by linking GMP decisions to risk, evidence, and context. This article helps teams decide when to act, what to document, and how to classify issues consistently before audits and regulatory inspections.




