Regulatory inspection trends continue to expose a recurring pattern across pharmaceutical manufacturing sites. FDA Form 483 summaries and EU inspection reports show that more than 30% of repeat GMP observations trace back to weak or poorly justified risk assessments. These findings highlight a critical reality: inspectors no longer accept risk management as a documentation exercise.
Instead, authorities increasingly evaluate how organisations use risk-based decisions in daily quality operations. Within this landscape, risk management in QMS has evolved into a proactive compliance tool that shapes inspection outcomes. Learn more about the broader framework of pharma quality management and how it supports inspection oversight.
Table of Contents
What Is Risk Management in QMS
Risk management within a Quality Management System refers to a structured, lifecycle-based approach for identifying, evaluating, controlling, and reviewing quality risks that can affect product quality and patient safety. Rather than reacting to deviations, organisations use this process to anticipate failures and act early.
In effective systems, risk management in QMS connects quality decisions across manufacturing, quality control, validation, and supply chain activities. As a result, risk considerations influence not only investigations and CAPA, but also change control, audit planning, and management review.
Regulatory Foundations of Risk Management in QMS
Global regulators align closely on expectations for risk-based quality systems. Authorities do not prescribe one single method, but they consistently expect structured, science-based decision-making.
Key regulatory foundations include:
- ICH Q9 principles for quality risk management
- EU GMP requirements for risk-based control of processes and systems
- FDA expectations for documented risk rationale during inspections
Together, these frameworks confirm that regulators expect organisations to explain why controls exist, not only demonstrate that they exist.
Risk-Based Thinking vs. Traditional Compliance
Traditional compliance relied heavily on checklist completion and procedural adherence. However, inspectors now look beyond document presence and focus on decision logic.
Risk-based thinking shifts the emphasis toward:
- Prioritising controls where failure impact is highest
- Allocating resources based on real risk exposure
- Documenting why certain risks are accepted, mitigated, or monitored
During inspections, authorities often ask why a control was chosen, not simply whether it was implemented.
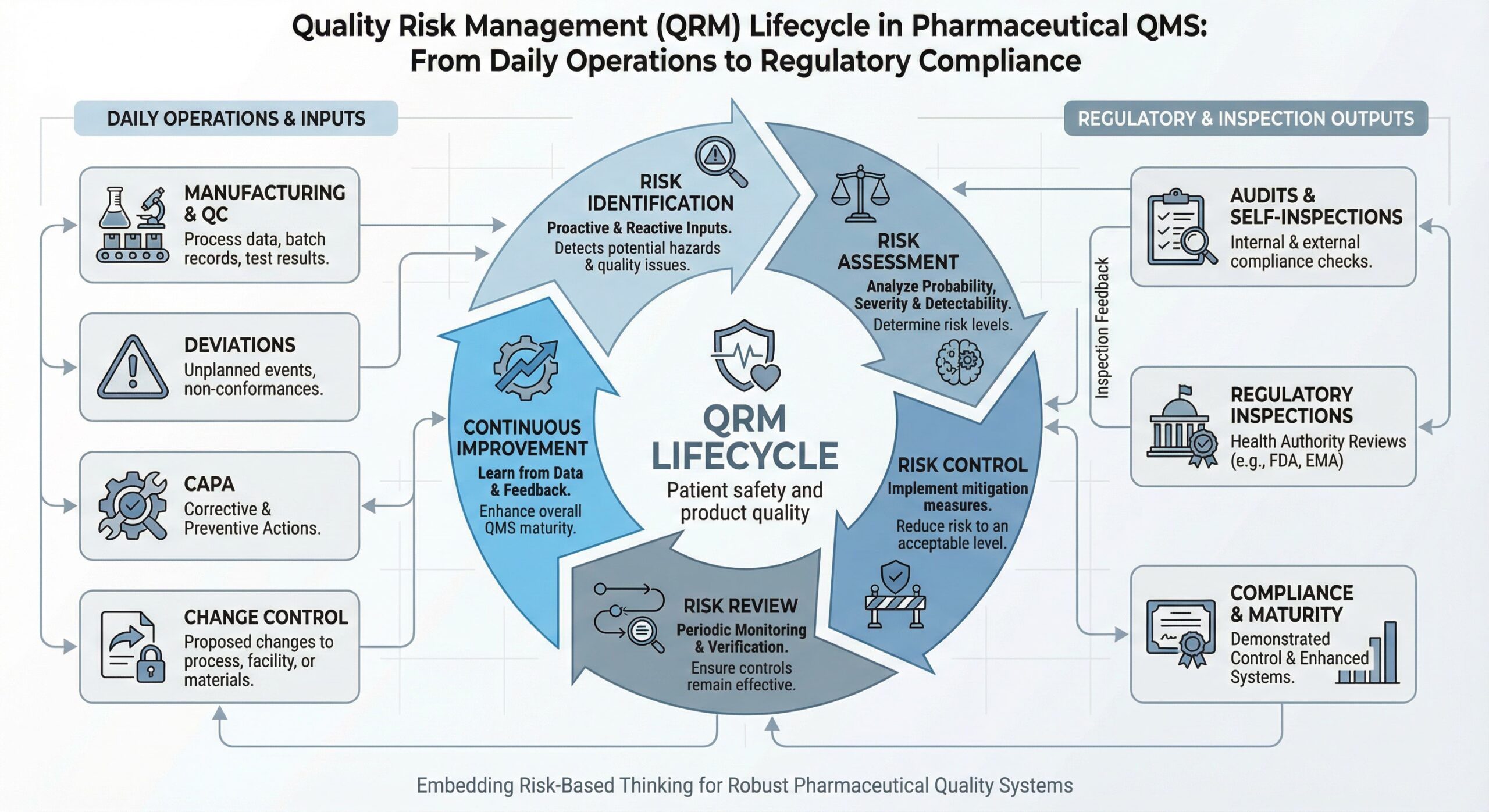
Key Elements of Effective Risk Management in a QMS
In a regulated pharmaceutical environment, quality risk management must operate as a core part of the Quality Management System. Regulators expect teams to apply risk-based thinking consistently across daily quality decisions. Therefore, organisations must embed risk evaluation into routine operations rather than treat it as standalone.
Moreover, inspectors assess how companies identify, assess, control, and review risks throughout the system lifecycle. As a result, a well-structured QMS risk assessment framework directly supports inspection readiness and compliance sustainability.
An effective approach relies on the following core elements:
- Structured Identification of Quality Risks Across Processes and Systems
- Defined Risk Control Actions and Mitigation Measures
- Integration With CAPA Risk Prioritisation, Change Control, and Audits
- Ongoing Lifecycle Risk Management and System Review
When these elements align, quality risk management within the QMS functions as a living control mechanism rather than a static compliance document.
Structured Identification of Quality Risks Across Processes and Systems
Quality teams must identify risks proactively across manufacturing operations, analytical testing, equipment design, data integrity controls, supplier qualification, and human factors. Instead of waiting for deviations, organisations should apply a risk-based approach in GMP activities from the outset.
Furthermore, inspectors review whether risk identification occurs systematically and whether teams use trend data or recurring observations to detect emerging risks early. When organisations identify risks only after major events, regulators often question overall system control. Therefore, early and structured identification remains a foundation of effective regulatory risk management.
Defined Risk Control Actions and Mitigation Measures
Risk evaluation must lead to proportionate and clearly defined control actions. These actions may include procedural safeguards, technical controls, enhanced monitoring, or additional validation activities.
Moreover, inspectors assess whether teams formally justify residual risk. When organisations implement controls without documented rationale, regulatory concerns frequently follow.
Integration With CAPA Risk Prioritisation, Change Control, and Audits
A mature Quality Management System links risk outputs directly to other quality processes. CAPA risk prioritisation should reflect both impact and recurrence potential. Likewise, change control risk evaluation must support approval decisions that affect validated states and product quality.
Additionally, audit programmes should focus on audit risk focus areas informed by historical performance and recurring system weaknesses. During inspections, regulators often challenge systems where risk assessments fail to influence investigation depth, CAPA scope, or audit planning.
Ongoing Lifecycle Risk Management and System Review
Risk management does not end after initial assessment. Instead, organisations must review risks based on lifecycle triggers such as trend analysis, recurring deviations, process changes, audit outcomes, and inspection feedback.
Furthermore, inspectors frequently cite outdated or static risk assessments as indicators of weak oversight. Therefore, continuous lifecycle risk management remains essential to maintain control and inspection readiness over time.
Risk Management During GMP Inspections
- During inspections, regulators rarely ask to see a single “risk file.” Instead, they trace how risk-based decision-making appears across quality systems and operational records.
Inspectors typically evaluate: - Whether investigations reflect meaningful risk evaluation logic
- How CAPAs address root causes and the likelihood of recurrence
- Whether change control decisions include documented risk impact assessment
- How audits prioritise high-risk processes, deviations, and emerging trends
Sites with mature quality risk governance demonstrate coherent, defensible decisions across departments, which not only strengthens inspection outcomes but also reduces escalation into system-level findings.
Final Words
Inspection trend analyses from FDA Form 483 databases and EU authority reports repeatedly show that sites with weak risk justification are more likely to receive repeat GMP observations compared to organisations with mature, risk-based systems. In several enforcement actions, regulators cited inadequate risk assessment as a contributing factor to warning letters.
For this reason, risk management in QMS no longer serves as supporting documentation. It functions as a predictive compliance mechanism that signals whether quality systems truly operate under control. Organisations that embed risk thinking into daily decisions not only reduce inspection findings, but also strengthen long-term regulatory trust.
FAQ
Inspectors review how quality risks influence real manufacturing and quality system decisions, such as deviation investigations, batch disposition, CAPA prioritisation, and change control approvals. They assess whether risk logic appears consistently across quality records, rather than existing only within formal risk assessment documents.
Inspection findings commonly arise when risk assessments remain generic, outdated, or disconnected from recurring deviations, ineffective CAPAs, or change control outcomes. Regulators often cite poor risk justification when organisations fail to explain why specific controls, mitigations, or priorities were selected within regulated operations.
Regulators associate inspection readiness with risk-based quality governance because it determines how manufacturing systems prevent recurrence, allocate quality resources, and maintain control over validated processes. Consistent risk-driven decisions demonstrate that quality systems remain under control between inspections, not only during regulatory visits.
References

Mahtab Shardi
Mahtab is a pharmaceutical professional with a Master’s degree in Physical Chemistry and over five years of experience in laboratory and QC roles. Mahtab contributes reliable, well-structured pharmaceutical content to Pharmuni, helping turn complex scientific topics into clear, practical insights for industry professionals and students.

Revalidation In Pharma (2026 guide): Meaning, Triggers, Frequency, And Requalification Differences
Revalidation protects product quality and business continuity by linking GMP decisions to risk, evidence, and context. This article helps teams decide when to act, what to document, and how to classify issues consistently before audits and regulatory inspections.

GMP Regulations in 2026: How Inspectors Assess Compliance and Control Systems
This article explains how pharmaceutical regulatory requirements shape inspection decisions, why GMP compliance gaps persist across manufacturing sites, and which operational controls, documentation practices, and risk-based measures support inspection readiness under global GMP standards.

WHO GMP in 2026: Inspection Readiness and Compliance Expectations
This article explains how global pharmaceutical GMP standards are applied during inspections, why operational gaps persist despite formal compliance, and how quality systems, contamination control, and risk-based execution shape regulatory inspection readiness across manufacturing operations.


