Requirements forMaterials in the Pharmaceutical Industry are stringent and must meet strict quality standards to ensure the safety and efficacy of the final product. Raw materials must be sourced from reputable suppliers and undergo thorough testing and verification to ensure they meet the necessary purity, potency, and quality specifications. These requirements are essential to prevent contamination, ensure consistency in manufacturing processes, and ultimately produce safe and effective pharmaceutical products for patients. The importance of raw materials in pharmaceutical manufacturing cannot be overstated, as they form the foundation of the entire manufacturing process. The quality and purity of raw materials directly impact the quality of the final product, making it crucial to source high-quality materials to maintain product integrity. Using substandard or contaminated raw materials can compromise the safety and efficacy of pharmaceutical products, leading to potential harm to patients and damage to the reputation of the manufacturer. By adhering to strict requirements and sourcing high-quality raw materials, pharmaceutical manufacturers can ensure the production of safe and effective medications that meet regulatory standards and benefit patients worldwide. |
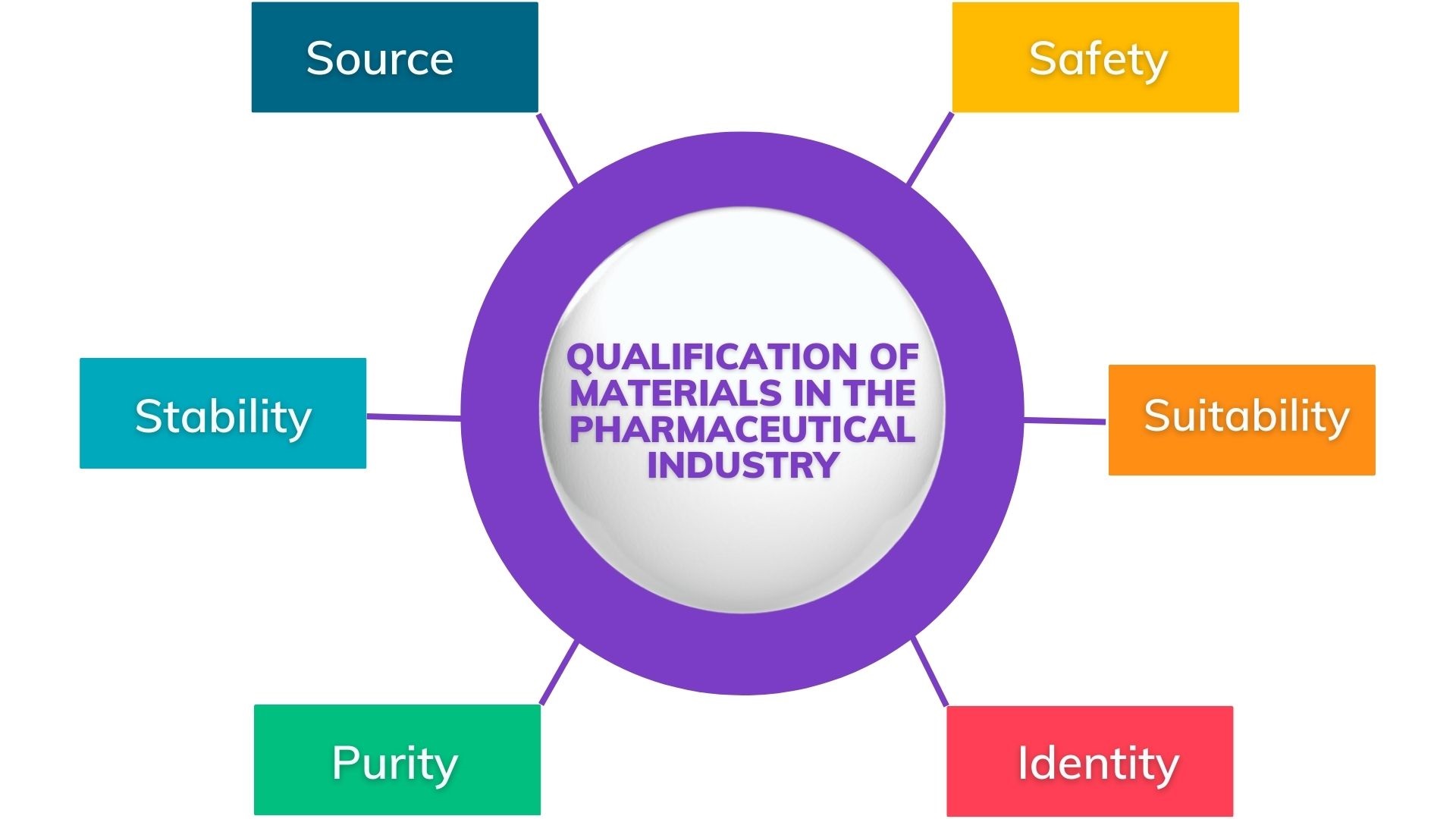
1️⃣ Qualification of Materials in the Pharmaceutical Industry
| Material qualification is a key step in the manufacturing of pharmaceuticals. It involves making sure that the materials used to make drugs meet high standards. This is important for ensuring patient safety. Different tests, like physical and chemical tests, are done to check for impurities in the materials. Material qualification also involves checking if the materials work well with the drug being made. It’s important to make sure that the materials don’t affect how well the drug works. It’s also important to make sure that the materials come from trusted suppliers. Things like certificates of analysis from suppliers are checked to make sure the materials are good quality. Material qualification is a process that needs to be done regularly. This is because materials can change over time. Checking materials regularly helps make sure that they always meet the required standards. Overall, material qualification is essential for making sure that pharmaceutical products are safe and effective. |
2️⃣ Essential Tips for Ensuring Pharmaceutical Grade Materials Meet the Highest Standards
Only work with suppliers who have a proven track record of providing high-quality materials that meet relevant pharmacopoeial standards.
Always obtain and review COAs from suppliers to verify material identity, purity, and compliance with specifications before use.
Define and document detailed specifications for each material, including acceptable ranges for critical quality attributes.
Conduct appropriate tests upon receipt of materials to verify their quality and ensure they meet predetermined specifications.
Store materials according to manufacturer recommendations to preserve their quality and prevent degradation or contamination.
Use materials based on their expiry dates to minimize the risk of using expired or degraded materials.
Maintain accurate records of material receipts, usage, and disposition to ensure traceability from supplier to finished product.
Periodically audit your suppliers to assess their quality systems, compliance, and ensure they continue to meet your requirements.
3️⃣ Different Materials in the Pharmaceutical Industry
3.1. Excipients in Pharmaceutical Formulation
Excipients are substances that are added to pharmaceuticals to serve various purposes such as adding bulk and ensuring stability, enhancing solubility and suspension, aiding in drug release and absorption, providing color and flavor, and protecting against degradation. They are essential in the development of dosage forms and can be derived from both organic and inorganic sources, often from natural sources like starch or cellulose. Excipients play a significant role in shaping and binding pharmaceutical formulations, influencing drug dissolution and bioavailability. Therefore, it is crucial to carefully select and test excipients for their safety and effectiveness. Ultimately, excipients are vital for maintaining the overall quality of medicines. |
3.2. API Sourcing and Quality Control
API sourcing involves finding reliable suppliers for raw materials of products. Quality control ensures consistency and adherence to standards in the manufacturing process. It is crucial to maintain high standards in API sourcing to prevent any compromise on product quality. Conducting thorough research and due diligence is essential in selecting reputable API suppliers. API sourcing directly impacts the overall quality and effectiveness of the final product. Quality control measures are put in place to detect and prevent any defects or inconsistencies in the products. Rigorous testing and inspections are carried out during the manufacturing process to ensure conformity to specifications. Compliance with regulatory standards is imperative in maintaining product integrity and safety. Effective quality control practices enhance consumer trust and confidence in the products. API sourcing and quality control help in minimizing production costs and waste while maximizing product efficiency. Continuous monitoring and evaluation are essential to identify areas for improvement in the sourcing and manufacturing process. Proper documentation and recordkeeping are essential in maintaining transparency and accountability in API sourcing and quality control processes. Excipients play a significant role in shaping and binding pharmaceutical formulations, influencing drug dissolution and bioavailability. Therefore, it is crucial to carefully select and test excipients for their safety and effectiveness. Ultimately, excipients are vital for maintaining the overall quality of medicines. |
3.3. Packaging Materials for Pharmaceuticals
Packaging materials for pharmaceuticals are crucial for ensuring the safety of medications. They must be durable to protect against external elements. The materials must also be resistant to moisture, light, and temperature changes. This is to ensure the stability and effectiveness of the medication. Additionally, packaging materials must be tamper-evident to prevent contamination or tampering. Many pharmaceutical companies use blister packs, bottles, and vials for packaging medications. These materials must also be child-resistant to prevent accidental ingestion. The packaging must provide clear and accurate information about the medication, including dosage instructions and expiration dates. Some medications require special packaging materials, such as those that need to be kept refrigerated. It is essential for pharmaceutical companies to comply with strict regulations when selecting packaging materials. Quality control measures must be in place to ensure the integrity of the packaging. Overall, the choice of packaging materials plays a critical role in maintaining the safety and efficacy of pharmaceutical products. Excipients play a significant role in shaping and binding pharmaceutical formulations, influencing drug dissolution and bioavailability. Therefore, it is crucial to carefully select and test excipients for their safety and effectiveness. Ultimately, excipients are vital for maintaining the overall quality of medicines. |
3.4. Management of Materials in the Pharmaceutical Industry
Pharmaceutical companies manage raw materials carefully to ensure product quality and safety. They source materials from reputable suppliers and test them thoroughly to meet strict standards. Raw materials are stored and handled properly to prevent contamination. Pharmaceutical companies track inventory levels and monitor supply chains to prevent stockouts or overstocking. They also maintain accurate records of material usage and dispose of expired or damaged materials properly. By managing materials effectively, pharmaceutical companies can produce high-quality medicines that meet regulatory standards and patient needs.
Conclusion
In conclusion, material qualification, excipients, API sourcing, packaging materials, and water quality all play critical roles in ensuring the quality, safety, and efficacy of pharmaceutical products. These factors must be carefully considered and monitored throughout the manufacturing process to prevent any compromise on product quality. By prioritizing quality control and adherence to strict standards, pharmaceutical manufacturers can produce medications that meet the highest standards of safety and efficacy for patients worldwide. Continuous monitoring, testing, and evaluation are essential to maintain product integrity and consumer trust. Overall, the quality of materials used in pharmaceutical manufacturing is essential in upholding the highest standards of patient care and product excellence.
References
1-Health products policy and standards
2-U.S. Food and Drug Administration (FDA) Current Good Manufacturing Practice Guidance for Human Drug Compounding Outsourcing Facilities Under Section 503B of the FD&C Act Guidance for Industry
https://www.fda.gov/media/88905/download
3- EMA Guidance on good manufacturing practice and good distribution practice: Questions and answers

Ershad Moradi
Content Marketing Specialist

GMP Environments in 2026: Inspection Readiness and Control Expectations
This article explains how inspectors evaluate GMP environments during regulatory inspections, why environmental findings often repeat, and how facility design, flow control, monitoring, and risk-based controls shape inspection readiness in real pharmaceutical manufacturing operations.

Adverse Event Reporting in 2026: Meaning, FAERS, EudraVigilance, And A Step-By-Step Guide
Adverse event reporting helps patients, healthcare professionals, and companies share safety concerns fast. Use the correct portal, submit complete case details, and send follow-up information. Clear reports support signal detection, risk review, and better pharmacovigilance decisions across systems globally today.

Master GxP Validation in 2026: Meaning, Key Steps, and Validated State Control
Auditors want evidence you can trace, not opinions you can explain. GxP validation links intended use, requirements, risk, and test results into one story. When you control changes and review performance, you keep the system inspection-ready every day on time.



